Korean J Physiol Pharmacol.
2019 Nov;23(6):475-482. 10.4196/kjpp.2019.23.6.475.
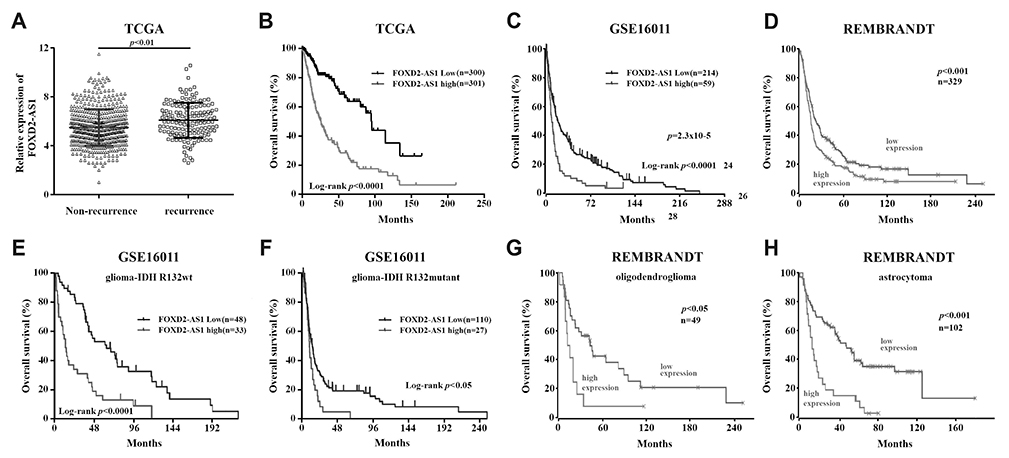
FoxD2-AS1 is a prognostic factor in glioma and promotes temozolomide resistance in a Oâ¶-methylguanine-DNA methyltransferase-dependent manner
- Affiliations
-
- 1Institute of Molecular Medicine, Life Science College, Zhejiang Chinese Medical University, Hangzhou 310053, Zhejiang Province, China. tiannanlux@126.com
- KMID: 2461040
- DOI: http://doi.org/10.4196/kjpp.2019.23.6.475
Abstract
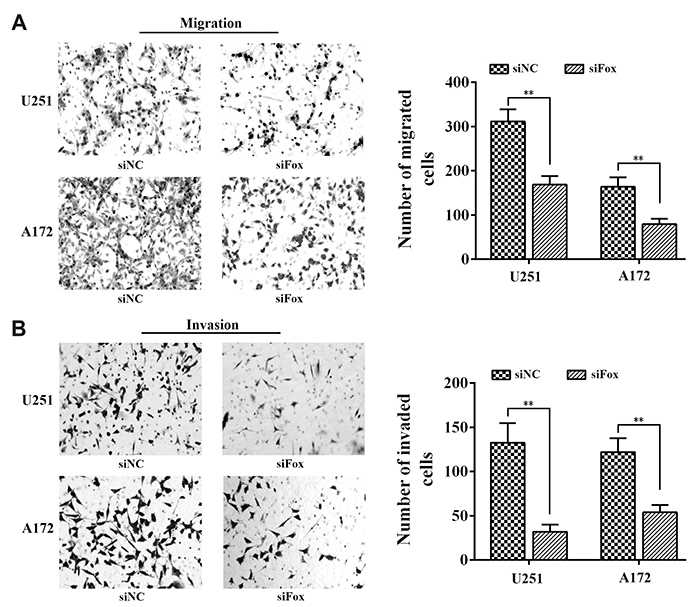
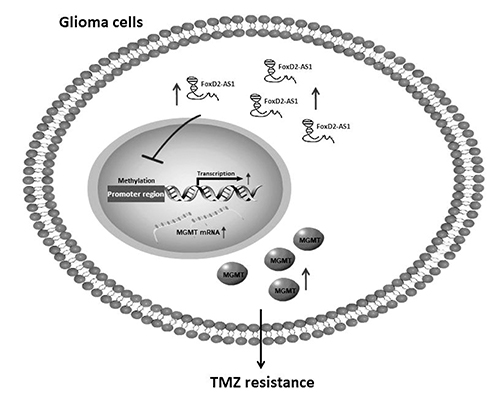
- Glioma is the most common brain tumor with a dismal prognosis. While temozolomide (TMZ) based chemotherapy significantly improves survival in glioma patients, resistance against this compound commonly leads to glioma treatment failure. Overexpression of long-noncoding RNA (LncRNA) FoxD2 adjacent opposite strand RNA 1 (FoxD2-AS1) was identified to promote glioma development, but the role in TMZ resistance remains unclear. In this paper, we found that FoxD2-AS1 was overexpressed in recurrent glioma, high FoxD2-AS1 expression was significantly correlated with poor patient outcome. Methylation of Oâ¶-methylguanine-DNA methyltransferase (MGMT) is significantly less frequent in high FoxD2-AS1 expression patients. Knockdown of FoxD2-AS1 decreased the proliferation, metastatic ability of glioma cells and promote the sensitivity to TMZ in glioma cells. Furthermore, knockdown of FoxD2-AS1 induced hypermethylation of the promoter region of MGMT. Our data suggested that FoxD2-AS1 is a clinical relevance LncRNA and mediates TMZ resistance by regulating the methylation status of the MGMT promoter region.
Keyword
MeSH Terms
Figure
Reference
-
1. Schijns VEJC, Pretto C, Strik AM, Gloudemans-Rijkers R, Deviller L, Pierre D, Chung J, Dandekar M, Carrillo JA, Kong XT, Fu BD, Hsu FPK, Hofman FM, Chen TC, Zidovetzki R, Bota DA, Stathopoulos A. Therapeutic immunization against glioblastoma. Int J Mol Sci. 2018; 19:E2540.
Article2. Lapointe S, Perry A, Butowski NA. Primary brain tumours in adults. Lancet. 2018; 392:432–446.
Article3. Chua J, Nafziger E, Leung D. Evidence-based practice: temozolomide beyond glioblastoma. Curr Oncol Rep. 2019; 21:30.
Article4. Roos WP, Batista LF, Naumann SC, Wick W, Weller M, Menck CF, Kaina B. Apoptosis in malignant glioma cells triggered by the temozolomide-induced DNA lesion O6-methylguanine. Oncogene. 2007; 26:186–197.5. Sarkaria JN, Kitange GJ, James CD, Plummer R, Calvert H, Weller M, Wick W. Mechanisms of chemoresistance to alkylating agents in malignant glioma. Clin Cancer Res. 2008; 14:2900–2908.
Article6. Storey K, Leder K, Hawkins-Daarud A, Swanson K, Ahmed AU, Rockne RC, Foo J. Glioblastoma recurrence and the role of O6-methylguanine-DNA methyltransferase promoter methylation. JCO Clin Cancer Inform. 2019; 3:1–12.7. Dunn J, Baborie A, Alam F, Joyce K, Moxham M, Sibson R, Crooks D, Husband D, Shenoy A, Brodbelt A, Wong H, Liloglou T, Haylock B, Walker C. Extent of MGMT promoter methylation correlates with outcome in glioblastomas given temozolomide and radiotherapy. Br J Cancer. 2009; 101:124–131.
Article8. van Nifterik KA, van den Berg J, van der Meide WF, Ameziane N, Wedekind LE, Steenbergen RD, Leenstra S, Lafleur MV, Slotman BJ, Stalpers LJ, Sminia P. Absence of the MGMT protein as well as methylation of the MGMT promoter predict the sensitivity for temozolomide. Br J Cancer. 2010; 103:29–35.
Article9. Huang FT, Chen WY, Gu ZQ, Zhuang YY, Li CQ, Wang LY, Peng JF, Zhu Z, Luo X, Li YH, Yao HR, Zhang SN. The novel long intergenic noncoding RNA UCC promotes colorectal cancer progression by sponging miR-143. Cell Death Dis. 2017; 8:e2778.
Article10. Sun Y, Hu B, Wang Q, Ye M, Qiu Q, Zhou Y, Zeng F, Zhang X, Guo Y, Guo L. Long non-coding RNA HOTTIP promotes BCL-2 expression and induces chemoresistance in small cell lung cancer by sponging miR-216a. Cell Death Dis. 2018; 9:85.
Article11. Xu N, Liu B, Lian C, Doycheva DM, Fu Z, Liu Y, Zhou J, He Z, Yang Z, Huang Q, Zeng H, Guo H. Long noncoding RNA AC003092.1 promotes temozolomide chemosensitivity through miR-195/TFPI-2 signaling modulation in glioblastoma. Cell Death Dis. 2018; 9:1139.
Article12. Du P, Zhao H, Peng R, Liu Q, Yuan J, Peng G, Liao Y. LncRNA-XIST interacts with miR-29c to modulate the chemoresistance of glioma cell to TMZ through DNA mismatch repair pathway. Biosci Rep. 2017; 37:BSR20170696.
Article13. Jiang P, Wang P, Sun X, Yuan Z, Zhan R, Ma X, Li W. Knockdown of long noncoding RNA H19 sensitizes human glioma cells to temozolomide therapy. Onco Targets Ther. 2016; 9:3501–3509.14. Liu Y, Xu N, Liu B, Huang Y, Zeng H, Yang Z, He Z, Guo H. Long noncoding RNA RP11-838N2.4 enhances the cytotoxic effects of temozolomide by inhibiting the functions of miR-10a in glioblastoma cell lines. Oncotarget. 2016; 7:43835–43851.
Article15. An Q, Zhou L, Xu N. Long noncoding RNA FoxD2-AS1 accelerates the gemcitabine-resistance of bladder cancer by sponging miR-143. Biomed Pharmacother. 2018; 103:415–420.16. Yang X, Duan B, Zhou X. Long non-coding RNA FoxD2-AS1 functions as a tumor promoter in colorectal cancer by regulating EMT and Notch signaling pathway. Eur Rev Med Pharmacol Sci. 2017; 21:3586–3591.17. Bao J, Zhou C, Zhang J, Mo J, Ye Q, He J, Diao J. Upregulation of the long noncoding RNA FoxD2-AS1 predicts poor prognosis in esophageal squamous cell carcinoma. Cancer Biomark. 2018; 21:527–533.18. Chen G, Sun W, Hua X, Zeng W, Yang L. Long non-coding RNA FoxD2-AS1 aggravates nasopharyngeal carcinoma carcinogenesis by modulating miR-363-5p/S100A1 pathway. Gene. 2018; 645:76–84.19. Shen F, Chang H, Gao G, Zhang B, Li X, Jin B. Long noncoding RNA FoxD2-AS1 promotes glioma malignancy and tumorigenesis via targeting miR-185-5p/CCND2 axis. J Cell Biochem. 2019; 120:9324–9336.20. Villanueva MT. Cell signalling: stuck in the middle of chemoresistance and metastasis. Nat Rev Clin Oncol. 2012; 9:490.21. Ni W, Xia Y, Bi Y, Wen F, Hu D, Luo L. FoxD2-AS1 promotes glioma progression by regulating miR-185-5P/HMGA2 axis and PI3K/AKT signaling pathway. Aging (Albany NY). 2019; 11:1427–1439.22. Li H, Chen L, Li JJ, Zhou Q, Huang A, Liu WW, Wang K, Gao L, Qi ST, Lu YT. miR-519a enhances chemosensitivity and promotes autophagy in glioblastoma by targeting STAT3/Bcl2 signaling pathway. J Hematol Oncol. 2018; 11:70.
Article23. Zheng X, Carstens JL, Kim J, Scheible M, Kaye J, Sugimoto H, Wu CC, LeBleu VS, Kalluri R. Epithelial-to-mesenchymal transition is dispensable for metastasis but induces chemoresistance in pancreatic cancer. Nature. 2015; 527:525–530.
Article24. Fischer KR, Durrans A, Lee S, Sheng J, Li F, Wong ST, Choi H, El Rayes T, Ryu S, Troeger J, Schwabe RF, Vahdat LT, Altorki NK, Mittal V, Gao D. Epithelial-to-mesenchymal transition is not required for lung metastasis but contributes to chemoresistance. Nature. 2015; 527:472–476.
Article25. Geldof AA, Rao BR. Doxorubicin treatment increases metastasis of prostate tumor (R3327-MatLyLu). Anticancer Res. 1988; 8:1335–1339.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Appraisal of re-irradiation for the recurrent glioblastoma in the era of MGMT promotor methylation
- Upregulation of lnc‑FOXD2‑AS1, CDC45, and CDK1 in patients with primary non‑M3 AML is associated with a worse prognosis
- A female adnexal tumor of probable Wolffian origin showing positive O-6-methylguanine-DNA methyltransferase methylation
- Role of Loss of O(6)-Methylguanine DNA Methyltransferase (MGMT) Expression in Non-Small Cell Lung Carcinomas (NSCLCs): with Reference to the Relationship with p53 Overexpression
- SP1-induced lncRNA MCF2L-AS1 promotes cisplatin resistance in ovarian cancer by regulating IGF2BP1/IGF2/MEK/ERK axis