Korean J Physiol Pharmacol.
2019 Nov;23(6):459-466. 10.4196/kjpp.2019.23.6.459.
Efficacy of evogliptin and cenicriviroc against nonalcoholic steatohepatitis in mice: a comparative study
- Affiliations
-
- 1College of Pharmacy, Woosuk University, Wanju 55338, Korea.
- 2Dong-A Socio Research Center, Dong-A ST Co., Ltd., Yongin 17073, Korea.
- 3College of Pharmacy, Chonbuk National University, Jeonju 54896, Korea. ejbae7@jbnu.ac.kr
- KMID: 2461038
- DOI: http://doi.org/10.4196/kjpp.2019.23.6.459
Abstract
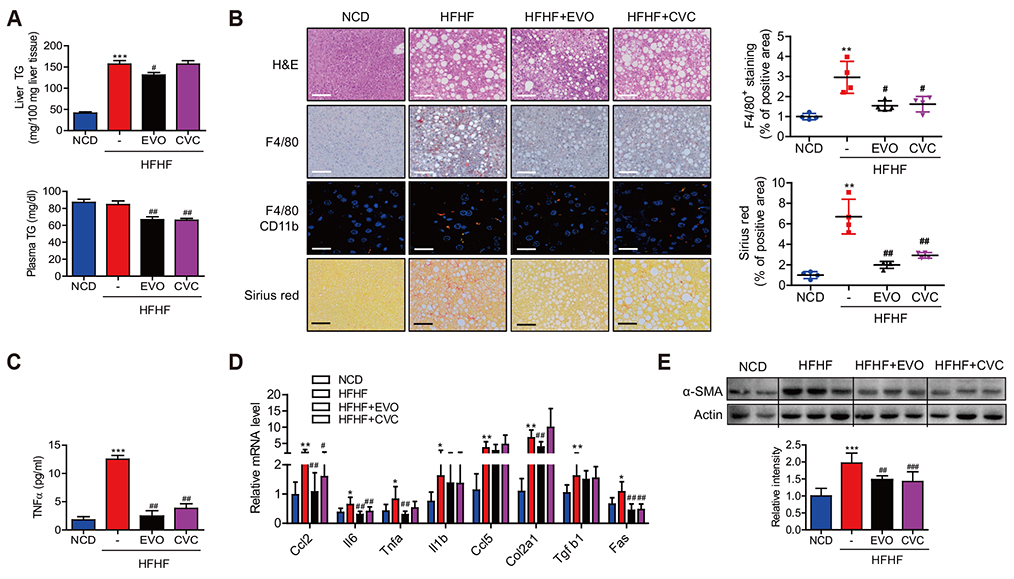
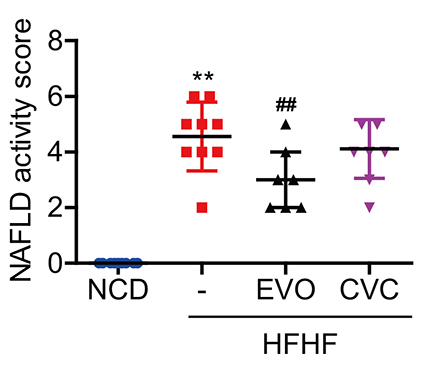
- Dipeptidyl peptidase (DPP)-4 inhibitors, or gliptins, are a class of oral hypoglycemic drugs that have been widely used as a second-line treatment for type 2 diabetes. Gliptins, which were introduced for clinical use a decade ago, have been shown to be beneficial against nonalcoholic fatty liver disease/nonalcoholic steatohepatitis (NASH) in animals and humans. Cenicriviroc (CVC), a dual antagonist of C-C chemokine receptor type 2 and 5, is currently under investigation against NASH and fibrosis. It was previously discovered that evogliptin (EVO) reduces hepatic steatosis in diet-induced obese animals but the effectiveness of EVO on NASH remains unexplored. Here, we compared the effectiveness of EVO and CVC against NASH and fibrosis in mice fed a high-fat and high-fructose diet (HFHF). Biochemical and histological analyses showed that mice fed a HFHF for 20 weeks developed severe hepatic steatosis and inflammation with mild fibrosis. Administration of EVO (0.2% wt/wt) for the last 8 weeks of HFHF feeding significantly reduced hepatic triglyceride accumulation, inflammation, and fibrosis as well as restored insulin sensitivity, as evidenced by lowered plasma insulin levels and the improvement in insulin tolerance test curves. Treatment of mice with CVC (0.1% wt/wt) inhibited hepatic inflammation and fibrogenesis with similar efficacy to that of EVO, without affecting hepatic steatosis. CVC treatment also reduced plasma insulin concentrations, despite no improvement in insulin tolerance. In conclusion, EVO administration efficiently ameliorated the development of NASH and fibrosis in HFHF-fed mice, corroborating its therapeutic potential.
Keyword
MeSH Terms
Figure
Reference
-
1. Jung YA, Choi YK, Jung GS, Seo HY, Kim HS, Jang BK, Kim JG, Lee IK, Kim MK, Park KG. Sitagliptin attenuates methionine/cholinedeficient diet-induced steatohepatitis. Diabetes Res Clin Pract. 2014; 105:47–57.
Article2. Klein T, Fujii M, Sandel J, Shibazaki Y, Wakamatsu K, Mark M, Yoneyama H. Linagliptin alleviates hepatic steatosis and inflammation in a mouse model of non-alcoholic steatohepatitis. Med Mol Morphol. 2014; 47:137–149.
Article3. Yilmaz Y, Yonal O, Deyneli O, Celikel CA, Kalayci C, Duman DG. Effects of sitagliptin in diabetic patients with nonalcoholic steatohepatitis. Acta Gastroenterol Belg. 2012; 75:240–244.4. Bae EJ. DPP-4 inhibitors in diabetic complications: role of DPP-4 beyond glucose control. Arch Pharm Res. 2016; 39:1114–1128.
Article5. Shirakawa J, Fujii H, Ohnuma K, Sato K, Ito Y, Kaji M, Sakamoto E, Koganei M, Sasaki H, Nagashima Y, Amo K, Aoki K, Morimoto C, Takeda E, Terauchi Y. Diet-induced adipose tissue inflammation and liver steatosis are prevented by DPP-4 inhibition in diabetic mice. Diabetes. 2011; 60:1246–1257.
Article6. Alam S, Ghosh J, Mustafa G, Kamal M, Ahmad N. Effect of sitagliptin on hepatic histological activity and fibrosis of nonalcoholic steatohepatitis patients: a 1-year randomized control trial. Hepat Med. 2018; 10:23–31.
Article7. Cui J, Philo L, Nguyen P, Hofflich H, Hernandez C, Bettencourt R, Richards L, Salotti J, Bhatt A, Hooker J, Haufe W, Hooker C, Brenner DA, Sirlin CB, Loomba R. Sitagliptin vs. placebo for nonalcoholic fatty liver disease: a randomized controlled trial. J Hepatol. 2016; 65:369–376.
Article8. Smits MM, Tonneijck L, Muskiet MH, Kramer MH, Pouwels PJ, Pieters-van den, Hoekstra T, Diamant M, van Raalte DH, Cahen DL. Twelve week liraglutide or sitagliptin does not affect hepatic fat in type 2 diabetes: a randomised placebo-controlled trial. Diabetologia. 2016; 59:2588–2593.
Article9. Tan X, Hu J. Evogliptin: a new dipeptidyl peptidase inhibitor for the treatment of type 2 diabetes. Expert Opin Pharmacother. 2016; 17:1285–1293.
Article10. McCormack PL. Evogliptin: first global approval. Drugs. 2015; 75:2045–2049.
Article11. Kim MK, Chae YN, Ahn GJ, Shin CY, Choi SH, Yang EK, Sohn YS, Son MH. Prevention and treatment effect of evogliptin on hepatic steatosis in high-fat-fed animal models. Arch Pharm Res. 2017; 40:268–281.
Article12. Thompson M, Saag M, DeJesus E, Gathe J, Lalezari J, Landay AL, Cade J, Enejosa J, Lefebvre E, Feinberg J. A 48-week randomized phase 2b study evaluating cenicriviroc versus efavirenz in treatment-naive HIV-infected adults with C-C chemokine receptor type 5-tropic virus. AIDS. 2016; 30:869–878.
Article13. Klibanov OM, Williams SH, Iler CA. Cenicriviroc, an orally active CCR5 antagonist for the potential treatment of HIV infection. Curr Opin Investig Drugs. 2010; 11:940–950.14. Friedman S, Sanyal A, Goodman Z, Lefebvre E, Gottwald M, Fischer L, Ratziu V. Efficacy and safety study of cenicriviroc for the treatment of non-alcoholic steatohepatitis in adult subjects with liver fibrosis: CENTAUR Phase 2b study design. Contemp Clin Trials. 2016; 47:356–365.
Article15. Saiman Y, Friedman SL. The role of chemokines in acute liver injury. Front Physiol. 2012; 3:213.
Article16. Seki E, de Minicis S, Inokuchi S, Taura K, Miyai K, van Rooijen N, Schwabe RF, Brenner DA. CCR2 promotes hepatic fibrosis in mice. Hepatology. 2009; 50:185–197.
Article17. Friedman SL, Ratziu V, Harrison SA, Abdelmalek MF, Aithal GP, Caballeria J, Francque S, Farrell G, Kowdley KV, Craxi A, Simon K, Fischer L, Melchor-Khan L, Vest J, Wiens BL, Vig P, Seyedkazemi S, Goodman Z, Wong VW, Loomba R, et al. A randomized, placebocontrolled trial of cenicriviroc for treatment of nonalcoholic steatohepatitis with fibrosis. Hepatology. 2018; 67:1754–1767.
Article18. Iogna Prat L, Tsochatzis EA. The effect of antidiabetic medications on non-alcoholic fatty liver disease (NAFLD). Hormones (Athens). 2018; 17:219–229.
Article19. Sanyal AJ, Chalasani N, Kowdley KV, McCullough A, Diehl AM, Bass NM, Neuschwander-Tetri BA, Lavine JE, Tonascia J, Unalp A, Van Natta M, Clark J, Brunt EM, Kleiner DE, Hoofnagle JH, Robuck PR. NASH CRN. Pioglitazone, vitamin E, or placebo for nonalcoholic steatohepatitis. N Engl J Med. 2010; 362:1675–1685.
Article20. Ratziu V. Novel pharmacotherapy options for NASH. Dig Dis Sci. 2016; 61:1398–1405.
Article21. Neuschwander-Tetri BA, Loomba R, Sanyal AJ, Lavine JE, Van Natta ML, Abdelmalek MF, Chalasani N, Dasarathy S, Diehl AM, Hameed B, Kowdley KV, McCullough A, Terrault N, Clark JM, Tonascia J, Brunt EM, Kleiner DE, Doo E. NASH Clinical Research Network. Farnesoid X nuclear receptor ligand obeticholic acid for non-cirrhotic, non-alcoholic steatohepatitis (FLINT): a multicentre, randomised, placebo-controlled trial. Lancet. 2015; 385:956–965.
Article22. Ratziu V, Harrison SA, Francque S, Bedossa P, Lehert P, Serfaty L, Romero-Gomez M, Boursier J, Abdelmalek M, Caldwell S, Drenth J, Anstee QM, Hum D, Hanf R, Roudot A, Megnien S, Staels B, Sanyal A. GOLDEN-505 Investigator Study Group. Elafibranor, an agonist of the peroxisome proliferator-activated receptor-α and -δ, induces resolution of nonalcoholic steatohepatitis without fibrosis worsening. Gastroenterology. 2016; 150:1147–1159.e5.23. Tomita K, Freeman BL, Bronk SF, LeBrasseur NK, White TA, Hirsova P, Ibrahim SH. CXCL10-mediates macrophage, but not other innate immune cells-associated inflammation in murine nonalcoholic steatohepatitis. Sci Rep. 2016; 6:28786.
Article24. Kim TH, Kim MK, Cheong YH, Chae YN, Lee Y, Ka SO, Jung IH, Shin CY, Bae EJ, Son MH. Hepatic role in an early glucose-lowering effect by a novel dipeptidyl peptidase 4 inhibitor, evogliptin, in a rodent model of type 2 diabetes. Eur J Pharmacol. 2016; 771:65–76.
Article25. Hübscher SG. Histological assessment of non-alcoholic fatty liver disease. Histopathology. 2006; 49:450–465.
Article26. Kleiner DE, Brunt EM, Van Natta M, Behling C, Contos MJ, Cummings OW, Ferrell LD, Liu YC, Torbenson MS, Unalp-Arida A, Yeh M, McCullough AJ, Sanyal AJ. Nonalcoholic Steatohepatitis Clinical Research Network. Design and validation of a histological scoring system for nonalcoholic fatty liver disease. Hepatology. 2005; 41:1313–1321.
Article27. Wang Z, Lee Y, Eun JS, Bae EJ. Inhibition of adipocyte inflammation and macrophage chemotaxis by butein. Eur J Pharmacol. 2014; 738:40–48.
Article28. Lefebvre E, Moyle G, Reshef R, Richman LP, Thompson M, Hong F, Chou HL, Hashiguchi T, Plato C, Poulin D, Richards T, Yoneyama H, Jenkins H, Wolfgang G, Friedman SL. Antifibrotic effects of the dual CCR2/CCR5 antagonist cenicriviroc in animal models of liver and kidney fibrosis. PLoS One. 2016; 11:e0158156.
Article29. Seki E, Schwabe RF. Hepatic inflammation and fibrosis: functional links and key pathways. Hepatology. 2015; 61:1066–1079.
Article30. Olivares M, Neyrinck AM, Pötgens SA, Beaumont M, Salazar N, Cani PD, Bindels LB, Delzenne NM. The DPP-4 inhibitor vildagliptin impacts the gut microbiota and prevents disruption of intestinal homeostasis induced by a Western diet in mice. Diabetologia. 2018; 61:1838–1848.
Article31. Seki E, De Minicis S, Gwak GY, Kluwe J, Inokuchi S, Bursill CA, Llovet JM, Brenner DA, Schwabe RF. CCR1 and CCR5 promote hepatic fibrosis in mice. J Clin Invest. 2009; 119:1858–1870.
Article32. Krenkel O, Puengel T, Govaere O, Abdallah AT, Mossanen JC, Kohlhepp M, Liepelt A, Lefebvre E, Luedde T, Hellerbrand C, Weiskirchen R, Longerich T, Costa IG, Anstee QM, Trautwein C, Tacke F. Therapeutic inhibition of inflammatory monocyte recruitment reduces steatohepatitis and liver fibrosis. Hepatology. 2018; 67:1270–1283.
Article33. Puengel T, Krenkel O, Kohlhepp M, Lefebvre E, Luedde T, Trautwein C, Tacke F. Differential impact of the dual CCR2/CCR5 inhibitor cenicriviroc on migration of monocyte and lymphocyte subsets in acute liver injury. PLoS One. 2017; 12:e0184694.
Article34. Schwabe RF, Bataller R, Brenner DA. Human hepatic stellate cells express CCR5 and RANTES to induce proliferation and migration. Am J Physiol Gastrointest Liver Physiol. 2003; 285:G949–G958.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Nonalcoholic Steatohepatitis
- Effects of the Combination of Evogliptin and Leucine on Insulin Resistance and Hepatic Steatosis in High-Fat Diet-Fed Mice
- Pathology of nonalcoholic steatohepatitis
- Pharmacological advances in the treatment of nonalcoholic fatty liver diseases : focused on global results of randomized controlled trials
- Clinical Predictors Reflecting the Pathologic Severity of Nonalcoholic Steatohepatitis in Patients with Nonalcoholic Fatty Liver