Korean Circ J.
2019 Nov;49(11):1010-1018. 10.4070/kcj.2019.0072.
Lipid-Core Plaque Assessed by Near-Infrared Spectroscopy and Procedure Related Microvascular Injury
- Affiliations
-
- 1Department of Cardiology, Ajou University School of Medicine, Suwon, Korea. yoonmh65@hanmail.net
- KMID: 2460525
- DOI: http://doi.org/10.4070/kcj.2019.0072
Abstract
- BACKGROUND AND OBJECTIVES
Microvascular damage due to distal embolization during percutaneous coronary intervention (PCI) is an important cause of periprocedural myocardial infarction. We assessed the lipid-core plaque using near-infrared spectroscopy (NIRS) and microvascular dysfunction invasively with the index of microcirculatory resistance (IMR) and evaluated their relationship.
METHODS
This study is pilot retrospective observational study. We analyzed 39 patients who performed NIRS before and after PCI, while fractional flow reserve, thermo-dilution coronary flow reserve (CFR) and IMR were measured after PCI. The maximum value of lipid core burden index (LCBI) for any of the 4-mm segments at the culprit lesion (culprit LCBI(4mm)) was calculated at the culprit lesion. We divided the patients into 2 groups using a cutoff of culprit LCBI(4mm) ≥500.
RESULTS
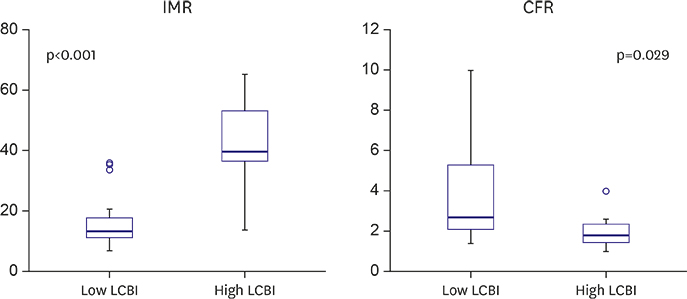
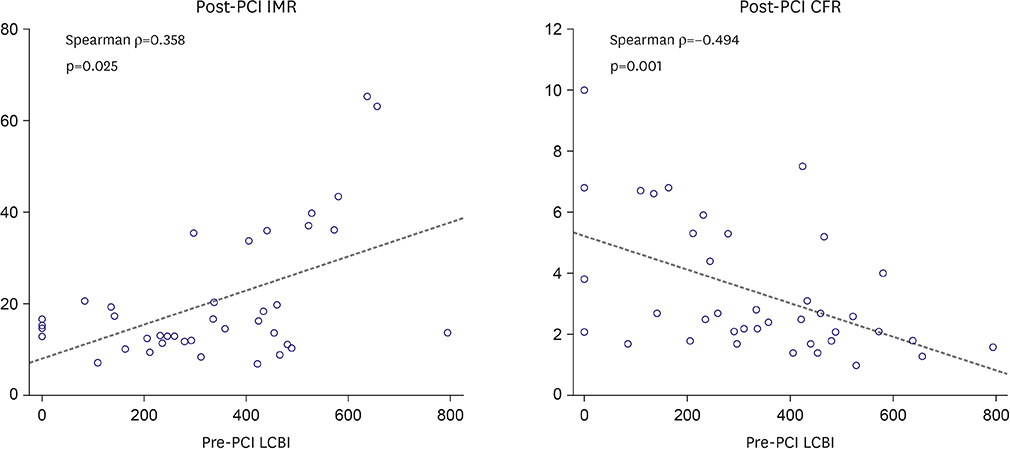
Mean pre-PCI LCBI was 333±196 and mean post-PCI IMR was 20±14 U. Post-PCI IMR was higher (15.6±7.3 vs. 42.6±17.6 U, p<0.001) and post-PCI CFR was lower (3.7±2.2 vs. 2.1±1.0, p=0.029) in the high LCBI group. Pre-PCI LCBI was positively correlated with post-PCI IMR (Ï=0.358, p=0.025) and negatively correlated with post-PCI CFR (Ï=−0.494, p=0.001). The incidence of microvascular dysfunction (IMR ≥25 U) was higher in the high LCBI group (9.4% vs. 85.7%, p<0.001). However, there were no significant differences in the incidences of creatine Kinase-MB (9.4% vs. 14.3%, p=0.563) and troponin-I elevation (12.5% vs. 14.3%, p=1.000).
CONCLUSIONS
A large lipid-core plaque at the "˜culprit' lesion is observed higher incidence of post-PCI microvascular dysfunction after PCI. Prospective study with adequate subject numbers will be needed.
Keyword
MeSH Terms
Figure
Cited by 1 articles
-
Glimpse of Relation between Imaging and Physiology
Jung Ho Heo
Korean Circ J. 2019;49(11):1019-1021. doi: 10.4070/kcj.2019.0295.
Reference
-
1. Henriques JP, Zijlstra F, Ottervanger JP, et al. Incidence and clinical significance of distal embolization during primary angioplasty for acute myocardial infarction. Eur Heart J. 2002; 23:1112–1117.
Article2. Prasad A, Singh M, Lerman A, Lennon RJ, Holmes DR Jr, Rihal CS. Isolated elevation in troponin T after percutaneous coronary intervention is associated with higher long-term mortality. J Am Coll Cardiol. 2006; 48:1765–1770.
Article3. Claessen BE, Maehara A, Fahy M, Xu K, Stone GW, Mintz GS. Plaque composition by intravascular ultrasound and distal embolization after percutaneous coronary intervention. JACC Cardiovasc Imaging. 2012; 5:S111–S118.
Article4. Idris H, Lo S, Shugman IM, et al. Varying definitions for periprocedural myocardial infarction alter event rates and prognostic implications. J Am Heart Assoc. 2014; 3:e001086.
Article5. Prati F, Pawlowski T, Gil R, et al. Stenting of culprit lesions in unstable angina leads to a marked reduction in plaque burden: a major role of plaque embolization? A serial intravascular ultrasound study. Circulation. 2003; 107:2320–2325.6. Herrmann J. Peri-procedural myocardial injury: 2005 update. Eur Heart J. 2005; 26:2493–2519.
Article7. Selvanayagam JB, Porto I, Channon K, et al. Troponin elevation after percutaneous coronary intervention directly represents the extent of irreversible myocardial injury: insights from cardiovascular magnetic resonance imaging. Circulation. 2005; 111:1027–1032.8. Lee SY, Mintz GS, Kim SY, et al. Attenuated plaque detected by intravascular ultrasound: clinical, angiographic, and morphologic features and post-percutaneous coronary intervention complications in patients with acute coronary syndromes. JACC Cardiovasc Interv. 2009; 2:65–72.9. Yamada R, Okura H, Kume T, et al. Target lesion thin-cap fibroatheroma defined by virtual histology intravascular ultrasound affects microvascular injury during percutaneous coronary intervention in patients with angina pectoris. Circ J. 2010; 74:1658–1662.
Article10. Lee T, Yonetsu T, Koura K, et al. Impact of coronary plaque morphology assessed by optical coherence tomography on cardiac troponin elevation in patients with elective stent implantation. Circ Cardiovasc Interv. 2011; 4:378–386.
Article11. Goldstein JA, Maini B, Dixon SR, et al. Detection of lipid-core plaques by intracoronary near-infrared spectroscopy identifies high risk of periprocedural myocardial infarction. Circ Cardiovasc Interv. 2011; 4:429–437.
Article12. Schuurman AS, Vroegindewey M, Kardys I, et al. Near-infrared spectroscopy-derived lipid core burden index predicts adverse cardiovascular outcome in patients with coronary artery disease during long-term follow-up. Eur Heart J. 2018; 39:295–302.
Article13. Fearon WF, Balsam LB, Farouque HM, et al. Novel index for invasively assessing the coronary microcirculation. Circulation. 2003; 107:3129–3132.
Article14. Moussa ID, Klein LW, Shah B, et al. Consideration of a new definition of clinically relevant myocardial infarction after coronary revascularization: an expert consensus document from the Society for Cardiovascular Angiography and Interventions (SCAI). J Am Coll Cardiol. 2013; 62:1563–1570.
Article15. Mintz GS, Nissen SE, Anderson WD, et al. American College of Cardiology clinical expert consensus document on standards for acquisition, measurement and reporting of intravascular ultrasound studies (IVUS). A report of the American College of Cardiology task force on clinical expert consensus documents. J Am Coll Cardiol. 2001; 37:1478–1492.16. Fearon WF, Farouque HM, Balsam LB, et al. Comparison of coronary thermodilution and Doppler velocity for assessing coronary flow reserve. Circulation. 2003; 108:2198–2200.
Article17. Kobayashi Y, Fearon WF. Invasive coronary microcirculation assessment--current status of index of microcirculatory resistance. Circ J. 2014; 78:1021–1028.18. Ahmed JM, Mintz GS, Weissman NJ, et al. Mechanism of lumen enlargement during intracoronary stent implantation: an intravascular ultrasound study. Circulation. 2000; 102:7–10.19. Hong YJ, Jeong MH, Choi YH, et al. Impact of plaque components on no-reflow phenomenon after stent deployment in patients with acute coronary syndrome: a virtual histology-intravascular ultrasound analysis. Eur Heart J. 2011; 32:2059–2066.
Article20. Jaross W, Neumeister V, Lattke P, Schuh D. Determination of cholesterol in atherosclerotic plaques using near infrared diffuse reflection spectroscopy. Atherosclerosis. 1999; 147:327–337.
Article21. Neumeister V, Scheibe M, Lattke P, Jaross W. Determination of the cholesterol-collagen ratio of arterial atherosclerotic plaques using near infrared spectroscopy as a possible measure of plaque stability. Atherosclerosis. 2002; 165:251–257.
Article22. Thygesen K, Alpert JS, Jaffe AS, et al. Third universal definition of myocardial infarction. Circulation. 2012; 126:2020–2035.
Article23. Ng MK, Yeung AC, Fearon WF. Invasive assessment of the coronary microcirculation: superior reproducibility and less hemodynamic dependence of index of microcirculatory resistance compared with coronary flow reserve. Circulation. 2006; 113:2054–2061.24. Cuisset T, Hamilos M, Melikian N, et al. Direct stenting for stable angina pectoris is associated with reduced periprocedural microcirculatory injury compared with stenting after pre-dilation. J Am Coll Cardiol. 2008; 51:1060–1065.
Article25. Brilakis ES, Abdel-Karim AR, Papayannis AC, et al. Embolic protection device utilization during stenting of native coronary artery lesions with large lipid core plaques as detected by near-infrared spectroscopy. Catheter Cardiovasc Interv. 2012; 80:1157–1162.
Article26. Stone GW, Maehara A, Muller JE, et al. Plaque characterization to inform the prediction and prevention of periprocedural myocardial infarction during percutaneous coronary intervention: the CANARY trial (Coronary Assessment by Near-infrared of Atherosclerotic Rupture-prone Yellow). JACC Cardiovasc Interv. 2015; 8:927–936.27. Lee BK, Koo BK, Nam CW, et al. Does pre-treatment with high dose atorvastatin prevent microvascular dysfunction after percutaneous coronary intervention in patients with acute coronary syndrome? Korean Circ J. 2016; 46:472–480.
Article28. Kim MC, Ahn Y, Cho JY, et al. Benefit of early statin initiation within 48 hours after admission in statin-naïve patients with acute myocardial infarction undergoing percutaneous coronary intervention. Korean Circ J. 2019; 49:419–433.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Two-channel Near-infrared Spectroscopic Analysis of Association of Paranoia Symptoms with Prefrontal Activation
- Clinical Application of Near Infrared Spectroscopy
- Plaque Characteristics Related to Reducing the Coronary Flow Reserve after Stenting: an Intravascular Ultrasound Study
- Clinical Application of Near-Infrared Spectroscopy in Neonates
- Identification of Coronary Healed Plaque Using a Combined HighDefinition 35–65 MHz Intravascular Ultrasound and Near-Infrared Spectroscopy