J Korean Ophthalmol Soc.
2019 Oct;60(10):975-981. 10.3341/jkos.2019.60.10.975.
Role of Hydrogen Sulfide in the Survival of Fibroblasts and Fibroblast-mediated Contraction of Collagen Gel
- Affiliations
-
- 1Department of Ophthalmology, Daegu Catholic University School of Medicine, Daegu, Korea. jwkim@cu.ac.kr
- KMID: 2460516
- DOI: http://doi.org/10.3341/jkos.2019.60.10.975
Abstract
- PURPOSE
To investigate the role of hydrogen sulfide in the survival and collagen gel contraction of cultured human Tenon's capsule fibroblasts (HTCFs).
METHODS
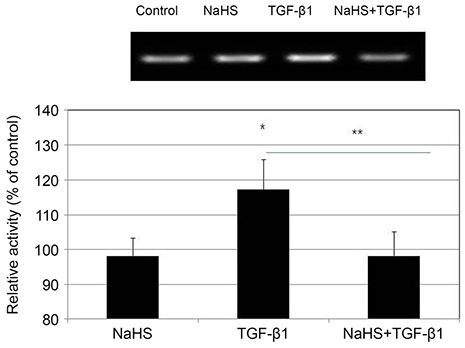
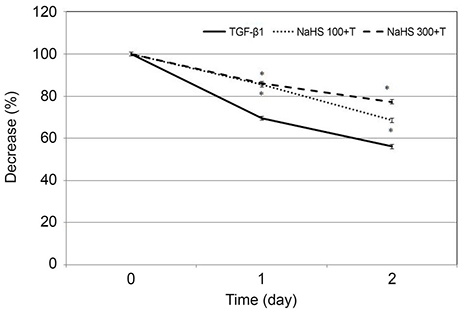
Primarily cultured HTCFs were exposed to 0, 100, 200, or 300 µM hydrogen sulfide (sodium hydrogen sulfide, NaHS) for 2 days. Cellular survival was assessed by MTT (3-[4, 5-dimethylthiazol-2-yl]-2, 5-diphenyltetrazolium bromide) assay. Degree of apoptosis was assessed with flow cytometry using annexin-V/propidium iodide double staining. To evaluate the effect of NaHS on cellular transdifferentiation, HTCFs were stimulated with 5 ng/mL TGF-β1 and the level of expression of α-smooth muscle actin (SMA) mRNA was assessed using reverse-transcription polymerase chain reaction. The cells were embedded in collagen gel, and the amount of gel contraction was measured.
RESULTS
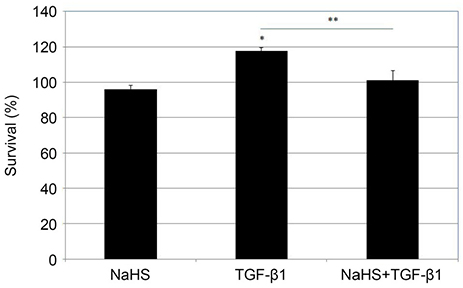
NaHS at 300 µM reduced HTCF survival (p = 0.013); NaHS at both 200 and 300 µM increased apoptosis in a dose-dependent manner (p = 0.013 and p = 0.016). TGF-β1 increased the expression of α-SMA mRNA (p = 0.041); co-treatment with 100 µM NaHS decreased TGF-β1-induced α-SMA mRNA expression (p = 0.039) and inhibited collagen gel contraction.
CONCLUSIONS
NaHS at high concentration reduced cellular survival and increased HTCF apoptosis. NaHS decreased TGF-β 1-induced increases in α-SMA mRNA expression and collagen gel contraction. Thus, hydrogen sulfide may suppress scar formation by inhibiting HTCF transdifferentiation and contraction of collagen gels.
MeSH Terms
Figure
Reference
-
1. Lama PJ, Fechtner RD. Antifibrotics and wound healing in glaucoma surgery. Surv Ophthalmol. 2003; 48:314–346.
Article2. Yoon PS, Singh K. Update on antifibrotic use in glaucoma surgery, including use in trabeculectomy and glaucoma drainage implants and combined cataract and glaucoma surgery. Curr Opin Ophthalmol. 2004; 15:141–146.
Article3. Cordeiro MF, Chang L, Lim KS, et al. Modulating conjunctival wound healing. Eye (Lond). 2000; 14 Pt 3B:536–547.
Article4. Mead AL, Wong TT, Cordeiro MF, et al. Evaluation of anti-TGF-β 2 antibody as a new postoperative anti-scarring agent in glaucoma surgery. Invest Ophthalmol Vis Sci. 2003; 44:3394–3401.5. Cordeiro MF, Reichel MB, Gay JA, et al. Transforming growth factor-beta 1, -beta 2, and -beta 3 in vivo: effects on normal and mitomycin C-modulated conjunctival scarring. Invest Ophthalmol Vis Sci. 1999; 40:1975–1982.6. Wang R. Two's company, three's a crowd: can H2S be the third endogenous gaseous transmitter? FASEB J. 2002; 16:1792–1798.7. Wang R. Physiological implications of hydrogen sulfide: a whiff exploration that blossomed. Physiol Rev. 2012; 92:791–896.
Article8. Parra O, Monsó E, Gallego M, Morera J. Inhalation of hydrogen sulphide: a case of subacute manifestations and long term sequelae. Br J Ind Med. 1991; 48:286–287.
Article9. Duong TX, Suruda AJ, Maier LA. Interstitial fibrosis following hydrogen sulfide exposure. Am J Ind Med. 2001; 40:221–224.
Article10. Fang LP, Lin Q, Tang CS, Liu XM. Hydrogen sulfide suppresses migration, proliferation and myofibroblast transdifferentiation of human lung fibroblasts. Pulm Pharmacol Ther. 2009; 22:554–561.
Article11. Sheng J, Shim W, Wei H, et al. Hydrogen sulphide suppresses human atrial fibroblast proliferation and transformation to myofibroblasts. J Cell Mol Med. 2013; 17:1345–1354.
Article12. Leslie M. Medicine. Nothing rotten about hydrogen sulfide's medical promise. Science. 2008; 320:1155–1157.
Article13. Li L, Whiteman M, Guan YY, et al. Characterization of a novel, water-soluble hydrogen sulfide releasing molecule (GYY4137): new insights into the biology of hydrogen sulfide. Circulation. 2008; 117:2351–2360.14. Baskar R, Bian J. Hydrogen sulfide gas has cell growth regulatory role. Eur J Pharmacol. 2011; 656:5–9.
Article15. Teunissen BE, Smeets PJ, Willemsen PH, et al. Activation of PPAR delta inhibits cardiac fibroblast proliferation and the transdifferentiation into myofibroblasts. Cardiovasc Res. 2007; 75:519–529.16. Björkerud S. Effects of transforming growth factor-beta 1 on human arterial smooth muscle cells in vitro. Arteriosclerosis Thromb. 1991; 11:892–902.
Article17. Skalli O, Schürch W, Seemayer T, et al. Myofibroblasts from diverse pathological settings are heterogeneous in their content of actin isoforms and intermediate filament proteins. Lab Invest. 1989; 60:275–285.18. Darby I, Skalli O, Gabbiani G. Alpha-smooth muscle actin is transiently expressed by myofibroblasts during experimental wound healing. Lab Invest. 1990; 63:21–29.19. Mosmann T. Rapid colorimetric assay for cellular growth and survival: application to proliferation and cytotoxicity assays. J Immunol Methods. 1983; 65:55–63.
Article20. Liu XD, Skold CM, Umino T, et al. Sodium nitroprusside augments human lung fibroblast collagen gel contraction independently of NO-cGMP pathway. Am J Physiol Lung Cell Mol Physiol. 2000; 278:L1032–L1038.21. Baskar R, Moore PK. Nuclear accumulation and up-regulation of p53 and its associated proteins after H2S treatment in human lung fibroblasts. J Cell Mol Med. 2008; 12:2297–2300.
Article22. Baskar R, Li L, Moore PK. Hydrogen sulfide-induces DNA damage and changes in apoptotic gene expression in human lung fibroblast cells. FASEB J. 2007; 21:247–255.
Article23. Shu DY, Lovicu FJ. Myofibroblast transdifferentiation: the dark force in ocular wound healing and fibrosis. Prog Retin Eye Res. 2017; 60:44–65.
Article24. Cordeiro MF. Beyond mitomycin: TGF-β and wound healing. Prog Retin Eye Res. 2002; 21:75–89.
Article25. Prendes MA, Harris A, Wirostko BM, et al. The role of transforming growth factor β in glaucoma and the therapeutic implications. Br J Ophthalmol. 2013; 97:680–686.
Article26. Cunliffe IA, Rees RC, Rennie IG. The effect of TGF-beta 1 and TGF-beta 2 on the proliferation of human Tenon's capsule fibroblasts in tissue culture. Acta Ophthalmol Scand. 1996; 74:31–35.
Article27. Browning AC, Alibhai A, McIntosh RS, et al. Effect of diabetes mellitus and hyperglycemia on the proliferation of human Tenon's capsule fibroblasts: implications for wound healing after glaucoma drainage surgery. Wound Repair Regen. 2005; 13:295–302.
Article28. Kay EP, Lee HK, Park KS, Lee SC. Indirect mitogenic effect of transforming growth factor-beta on cell proliferation of subconjunctival fibroblasts. Invest Ophthalmol Vis Sci. 1998; 39:481–486.29. Denk PO, Hoppe J, Hoppe V, Knorr M. Effect of growth factors on the activation of human Tenon's capsule fibroblasts. Curr Eye Res. 2003; 27:35–44.
Article30. Desmouliére A, Geinoz A, Gabbiani F, Gabbiani G. Transforming growth factor-β induces α-smooth muscle actin expression in granulation tissue myofibroblasts and in quiescent and growing cultured fibroblasts. J Cell Biol. 1993; 122:103–111.31. Olson ER, Naugle JE, Zhang X, et al. Inhibition of cardiac fibroblast proliferation and myofibroblast differentiation by resveratrol. Am J Physiol Heart Circ Physiol. 2005; 288:H1131–H1138.
Article32. Cordeiro MF, Gay JA, Khaw PT. Human anti-transforming growth factor-beta2 antibody: a new glaucoma anti-scarring agent. Invest Ophthalmol Vis Sci. 1999; 40:2225–2234.33. Meyer-ter-Vehn T, Sieprath S, Katzenbergeret B, et al. Contractility as a prerequisite for TGF-β–induced myofibroblast transdifferentiation in human tenon fibroblasts. Invest Ophthalmol Vis Sci. 2006; 47:4895–4904.
Article34. Desmoulière A, Redard M, Darby I, Gabbiani G. Apoptosis mediates the decrease in cellularity during the transition between granulation tissue and scar. Am J Pathol. 1995; 146:56–66.35. Kottler UB, Jünemann AG, Aigner T, et al. Comparative effects of TGF-beta 1 and TGF-beta 2 on extracellular matrix production, proliferation, migration, and collagen contraction of human Tenon's capsule fibroblasts in pseudoexfoliation and primary open-angle glaucoma. Exp Eye Res. 2005; 80:121–134.36. Pierce GF, Mustoe TA, Lingelbach J, et al. Platelet-derived growth factor and transforming growth factor-β enhance tissue repair activities by unique mechanisms. J Cell Biol. 1989; 109:429–440.37. Li Z, Bergen TV, Van de, et al. Inhibition of vascular endothelial growth factor reduces scar formation after glaucoma filtering surgery. Invest Ophthalmol Vis Sci. 2009; 50:5217–5225.38. Zhou X, An G, Chen J. Inhibitory effects of hydrogen sulphide on pulmonary fibrosis in smoking rats via attenuation of oxidative stress and inflammation. J Cell Mol Med. 2014; 18:1098–1103.39. Kansanen E, Kuosmanen SM, Leinonen H, Levonen AL. The Keap1-Nrf2 pathway: mechanisms of activation and dysregulation in cancer. Redox Biol. 2013; 1:45–49.
Article40. Kim JW, Kim SK, Song IH, Kim IT. Mitomycin C-induced apoptosis in cultured human tenon's capsule fibroblasts. Korean J Ophthalmol. 1999; 13:7–15.
Article41. Lee SE, Kim JW. Effects of valproic acid on the survival of human tennon's capsule fibroblasts. J Korean Ophthalmol Soc. 2018; 59:1056–1061.
Article42. Seibold LK, Sherwood MB, Kahook MY. Wound modulation after filtration surgery. Surv Ophthalmol. 2012; 57:530–550.
Article43. Zada M, Pattamatta U, White A. Modulation of fibroblasts in conjunctival wound healing. Ophthalmology. 2018; 125:179–192.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Effect of Nitric Oxide on the Migration and Fibroblast-mediated Contraction of Collagen Gels
- Collagen Gel Contraction by Cultured Fibroblasts Derived from Normal Skin, Oral Mucosa, and Hypertrophic Scar
- The Effect of Anticancerous Drug in the Fiboblast-Mediated Collagen Matrix Contraction
- Role of IL-5-activated eosinophils on collagen gel contraction by lung fibroblasts
- Effect of Amniotic Membrane Extract on the Tenon's Capsule Fibroblasts