Perinatology.
2019 Sep;30(3):117-125. 10.14734/PN.2019.30.3.117.
SYM 2081 Exerts Neuroprotective Effect Modulating Anti-Apoptosis in the Hypoxic-Ischemic Brain Injury of Neonatal Rats
- Affiliations
-
- 1Department of Pediatrics, Catholic University of Daegu School of Medicine, Daegu, Korea. wootykim@hanmail.net
- 2Department of Pediatrics, Kyungpook National University School of Medicine, Daegu, Korea.
- KMID: 2460024
- DOI: http://doi.org/10.14734/PN.2019.30.3.117
Abstract
OBJECTIVE
SYM 2081 ((2S,4R)-4-methylglutamic acid), an agonist and functional antagonist of the kainite receptor, has shown a neuroprotective effect in neurodegenerative diseases in adults but little is known concerning perinatal hypoxic-ischemic encephalopathy. This study is designed to evaluate whether SYM 2081 has preventive mechanisms via anti-apoptosis to gain further insight into neuroprotective roles of SYM 2081.
METHODS
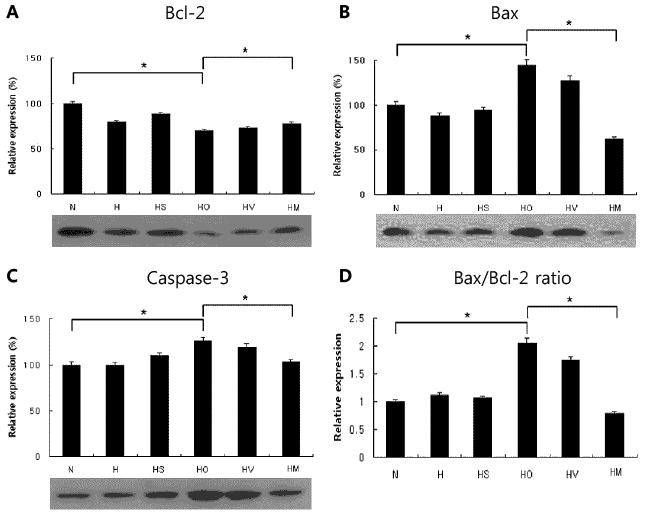
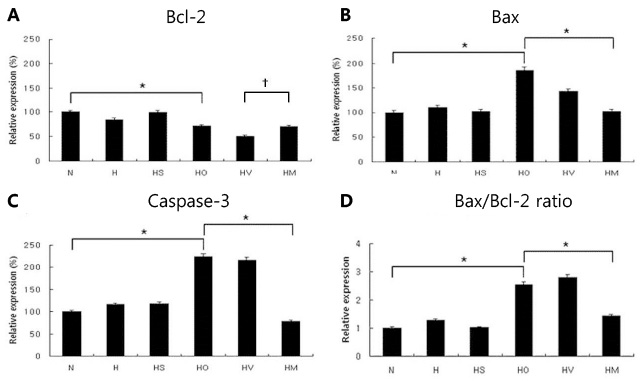
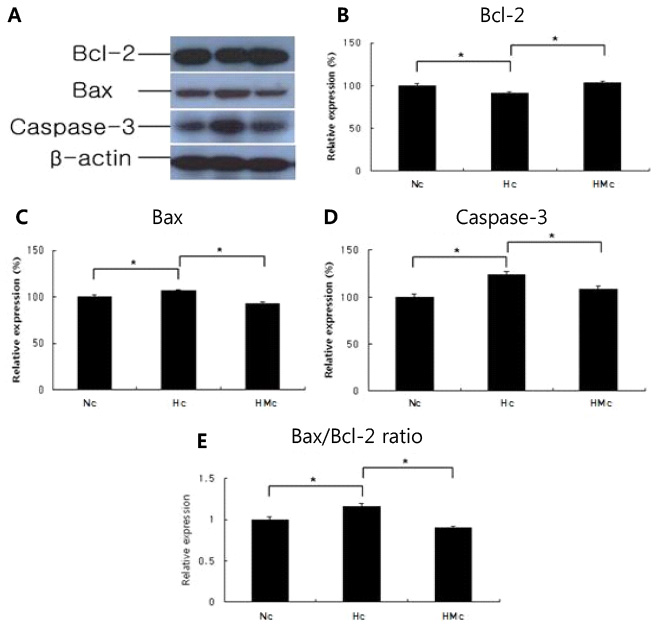
In an in vivo animal model, the left carotid artery was ligated in 7-day-old Sprague-Dawley rat pups. The pups were divided into six groups: normoxia (N), hypoxia (8% Oâ‚‚, 92% Nâ‚‚) (H), H with shamoperation, H with operation (HO), HO treated with vehicle and HO treated with SYM 2081. In an in vitro model, the cultured embryonic cortical neuronal cells were divided into three groups: normoxia (95% air, 5% COâ‚‚), hypoxia (94% Nâ‚‚, 5% COâ‚‚) (Hc), and Hc treated with SYM 2081 before a hypoxic insult. Apoptosis was assessed using western blots and real-time polymerase chain reaction with Bcl-2, Bax, and caspase-3 antibodies and mRNAs.
RESULTS
In both in vitro and in vivo studies, Bcl-2 expression increased, whereas expressions of Bax, caspase-3, and the ratio of Bax/Bcl-2 were reduced with SYM 2081 treatment resulting in improved cell survival.
CONCLUSION
This study showed that SYM 2081 exerts a neuroprotective effect against hypoxic-ischemic injury through modulating apoptotic signaling pathways.
Keyword
MeSH Terms
-
Adult
Animals
Anoxia
Antibodies
Apoptosis
Blotting, Western
Brain Injuries*
Brain*
Carotid Arteries
Caspase 3
Cell Survival
Humans
Hypoxia-Ischemia, Brain
In Vitro Techniques
Models, Animal
Neurodegenerative Diseases
Neurons
Neuroprotective Agents*
Rats*
Rats, Sprague-Dawley
Real-Time Polymerase Chain Reaction
RNA, Messenger
Antibodies
Caspase 3
Neuroprotective Agents
RNA, Messenger
Figure
Reference
-
1. Wu Q, Chen W, Sinha B, Tu Y, Manning S, Thomas N, et al. Neuroprotective agents for neonatal hypoxic-ischemic brain injury. Drug Discov Today. 2015; 20:1372–1381.
Article2. Tagin MA, Woolcott CG, Vincer MJ, Whyte RK, Stinson DA. Hypothermia for neonatal hypoxic ischemic encephalopathy: an updated systematic review and meta-analysis. Arch Pediatr Adolesc Med. 2012; 166:558–566.
Article3. Higgins RD, Raju T, Edwards AD, Azzopardi DV, Bose CL, Clark RH, et al. Hypothermia and other treatment options for neonatal encephalopathy: an executive summary of the Eunice Kennedy Shriver NICHD workshop. J Pediatr. 2011; 159:851–858.
Article4. Northington FJ, Chavez-Valdez R, Martin LJ. Neuronal cell death in neonatal hypoxia-ischemia. Ann Neurol. 2011; 69:743–758.e1.
Article5. Yang LJ, Ma DQ, Cui H. Proteomic analysis of immature rat pups brain in response to hypoxia and ischemia challenge. Int J Clin Exp Pathol. 2014; 7:4645–4660.6. Delivoria-Papadopoulos M, Ashraf QM, Ara J, Mishra OP. Nuclear mechanisms of hypoxic cerebral injury in the newborn: the role of caspases. Semin Perinatol. 2008; 32:334–343.7. Yuan J, Lipinski M, Degterev A. Diversity in the mechanisms of neuronal cell death. Neuron. 2003; 40:401–413.
Article8. Youle RJ, Strasser A. The BCL-2 protein family: opposing activities that mediate cell death. Nat Rev Mol Cell Biol. 2008; 9:47–59.
Article9. Douglas-Escobar M, Weiss MD. Biomarkers of brain injury in the premature infant. Front Neurol. 2012; 3:185.
Article10. Sims NR, Muyderman H. Mitochondria, oxidative metabolism and cell death in stroke. Biochim Biophys Acta. 2010; 1802:80–91.
Article11. Gulyaeva NV. Non-apoptotic functions of caspase-3 in nervous tissue. Biochemistry (Mosc). 2003; 68:1171–1180.
Article12. Cheng Y, Deshmukh M, D'Costa A, Demaro JA, Gidday JM, Shah A, et al. Caspase inhibitor affords neuroprotection with delayed administration in a rat model of neonatal hypoxic-ischemic brain injury. J Clin Invest. 1998; 101:1992–1999.
Article13. Lipton SA, Choi YB, Pan ZH, Lei SZ, Chen HS, Sucher NJ, et al. A redox-based mechanism for the neuroprotective and neurodestructive effects of nitric oxide and related nitroso-compounds. Nature. 1993; 364:626–632.
Article14. Mueller-Burke D, Koehler RC, Martin LJ. Rapid NMDA receptor phosphorylation and oxidative stress precede striatal neurodegeneration after hypoxic ischemia in newborn piglets and are attenuated with hypothermia. Int J Dev Neurosci. 2008; 26:67–76.15. Benveniste M, Wilhelm J, Dingledine RJ, Mott DD. Subunit-dependent modulation of kainate receptors by muscarinic acetylcholine receptors. Brain Res. 2010; 1352:61–69.
Article16. Lerma J, Marques JM. Kainate receptors in health and disease. Neuron. 2013; 80:292–311.
Article17. Sihra TS, Flores G, Rodríguez-Moreno A. Kainate receptors: multiple roles in neuronal plasticity. Neuroscientist. 2014; 20:29–43.18. Lee JH, Lee EJ, Jang YY, Jeong JE, Chung HL, Kim WT. Neuroprotective effects of SYM 2081, targeting kainate receptors in the neonatal hypoxic-ischemic brain injury. Perinatology. 2016; 27:149–157.
Article19. Rice JE 3rd, Vannucci RC, Brierley JB. The influence of immaturity on hypoxic-ischemic brain damage in the rat. Ann Neurol. 1981; 9:131–141.
Article20. Brewer GJ. Isolation and culture of adult rat hippocampal neurons. J Neurosci Methods. 1997; 71:143–155.
Article21. Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) method. Methods. 2001; 25:402–408.22. Proskuryakov SY, Konoplyannikov AG, Gabai VL. Necrosis: a specific form of programmed cell death? Exp Cell Res. 2003; 283:1–16.
Article23. Carloni S, Carnevali A, Cimino M, Balduini W. Extended role of necrotic cell death after hypoxia-ischemia-induced neurodegeneration in the neonatal rat. Neurobiol Dis. 2007; 27:354–361.
Article24. Kiraz Y, Adan A, Kartal Yandim M, Baran Y. Major apoptotic mechanisms and genes involved in apoptosis. Tumour Biol. 2016; 37:8471–8486.
Article25. Kajta M. Apoptosis in the central nervous system: mechanisms and protective strategies. Pol J Pharmacol. 2004; 56:689–700.26. Arthur DB, Georgi S, Akassoglou K, Insel PA. Inhibition of apoptosis by P2Y2 receptor activation: novel pathways for neuronal survival. J Neurosci. 2006; 26:3798–3804.
Article27. Natale JE, Cheng Y, Martin LJ. Thalamic neuron apoptosis emerges rapidly after cortical damage in immature mice. Neuroscience. 2002; 112:665–676.
Article28. Northington FJ, Zelaya ME, O'Riordan DP, Blomgren K, Flock DL, Hagberg H, et al. Failure to complete apoptosis following neonatal hypoxia-ischemia manifests as “continuum” phenotype of cell death and occurs with multiple manifestations of mitochondrial dysfunction in rodent forebrain. Neuroscience. 2007; 149:822–833.
Article29. Chalak LF, Sánchez PJ, Adams-Huet B, Laptook AR, Heyne RJ, Rosenfeld CR. Biomarkers for severity of neonatal hypoxic-ischemic encephalopathy and outcomes in newborns receiving hypothermia therapy. J Pediatr. 2014; 164:468–474.e1.
Article30. Carloni S, Buonocore G, Balduini W. Protective role of autophagy in neonatal hypoxia-ischemia induced brain injury. Neurobiol Dis. 2008; 32:329–339.
Article31. Jones KA, Wilding TJ, Huettner JE, Costa AM. Desensitization of kainate receptors by kainate, glutamate and diastereomers of 4-methylglutamate. Neuropharmacology. 1997; 36:853–863.
Article32. Donevan SD, Beg A, Gunther JM, Twyman RE. The methylglutamate, SYM 2081, is a potent and highly selective agonist at kainate receptors. J Pharmacol Exp Ther. 1998; 285:539–545.33. Carver JM, Mansson PE, Cortes-Burgos L, Shu J, Zhou LM, Howe JR, et al. Cytotoxic effects of kainate ligands on HEK cell lines expressing recombinant kainate receptors. Brain Res. 1996; 720:69–74.
Article34. Zhou LM, Gu ZQ, Costa AM, Yamada KA, Mansson PE, Giordano T, et al. (2S,4R)-4-methylglutamic acid (SYM 2081): a selective, high-affinity ligand for kainate receptors. J Pharmacol Exp Ther. 1997; 280:422–427.35. Andrade-Talavera Y, Duque-Feria P, Negrete-Díaz JV, Sihra TS, Flores G, Rodríguez-Moreno A. Presynaptic kainate receptor-mediated facilitation of glutamate release involves Ca2+ -calmodulin at mossy fiber-CA3 synapses. J Neurochem. 2012; 122:891–899.36. Alt A, Weiss B, Ogden AM, Knauss JL, Oler J, Ho K, et al. Pharmacological characterization of glutamatergic agonists and antagonists at recombinant human homomeric and heteromeric kainate receptors in vitro. Neuropharmacology. 2004; 46:793–806.
Article37. Hernández-Jiménez M, Sacristán S, Morales C, García-Villanueva M, García-Fernández E, Alcázar A, et al. Apoptosis-related proteins are potential markers of neonatal hypoxic-ischemic encephalopathy (HIE) injury. Neurosci Lett. 2014; 558:143–148.
Article38. Sun MY, Cui KJ, Yu MM, Zhang H, Peng XL, Jiang H. Bax inhibiting peptide reduces apoptosis in neonatal rat hypoxic-ischemic brain damage. Int J Clin Exp Pathol. 2015; 8:14701–14708.39. Northington FJ, Ferriero DM, Flock DL, Martin LJ. Delayed neurodegeneration in neonatal rat thalamus after hypoxia-ischemia is apoptosis. J Neurosci. 2001; 21:1931–1938.
Article40. Blomgren K, Zhu C, Wang X, Karlsson JO, Leverin AL, Bahr BA, et al. Synergistic activation of caspase-3 by m-calpain after neonatal hypoxia-ischemia: a mechanism of "pathological apoptosis"? J Biol Chem. 2001; 276:10191–10198.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Neuroprotective Effects of SYM 2081, Targeting Kainate Receptors in the Neonatal Hypoxic-Ischemic Brain Injury
- Neuroprotective Effect of Growth Hormone in Neonatal Rat with Hypoxic Ischemic Brain Injury
- Taurine exerts neuroprotective effects via anti-apoptosis in hypoxic-ischemic brain injury in neonatal rats
- Neuroprotective effects of resveratrol via anti-apoptosis on hypoxic-ischemic brain injury in neonatal rats
- Neuroprotective Effect of Dizocilpine (MK-801) via Anti-apoptosis on Hypoxic-ischemic Brain Injury in Neonatal Rats