Nat Prod Sci.
2019 Sep;25(3):181-199. 10.20307/nps.2019.25.3.181.
Ethnobotany, Phytochemistry, and Pharmacology of Angelica decursiva Fr. et Sav.
- Affiliations
-
- 1Department of Chemistry and Biochemistry, Faculty of Arts and Science, Concordia University, 7141 Sherbrooke St. W., Montreal, Quebec, Canada.
- 2Department of Biology, Faculty of Arts and Science, Concordia University, 7141 Sherbrooke St. W., Montreal, Quebec, Canada.
- 3Centre for Structural and Functional Genomic, Dept. of Biology, Faculty of Arts and Science, Concordia University, 7141 Sherbrooke St. W., Montreal, QC, Canada.
- 4Department of Food and Life Science, Pukyong National University, Busan 48513, Republic of Korea. choijs@pknu.ac.kr
- 5Department of Food Science and Human Nutrition, Chonbuk National University, Jeonju 54896, Republic of Korea.
- KMID: 2459959
- DOI: http://doi.org/10.20307/nps.2019.25.3.181
Abstract
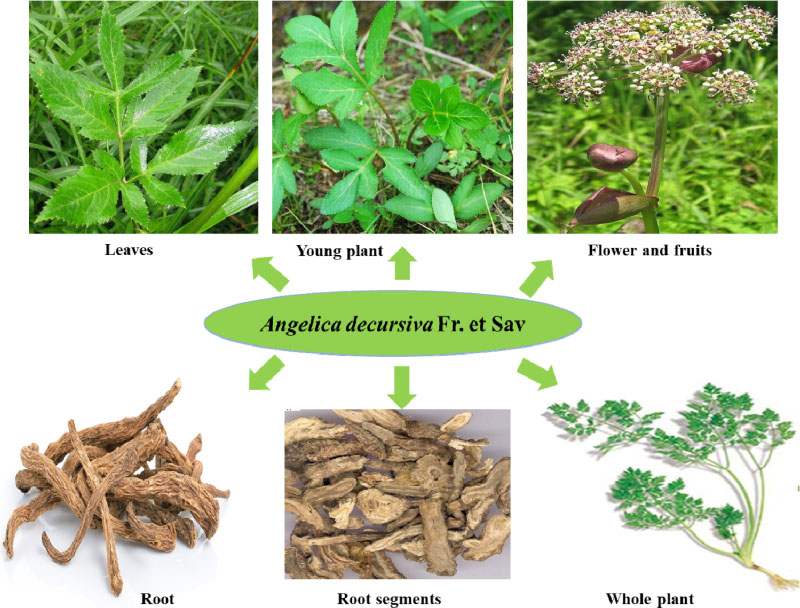
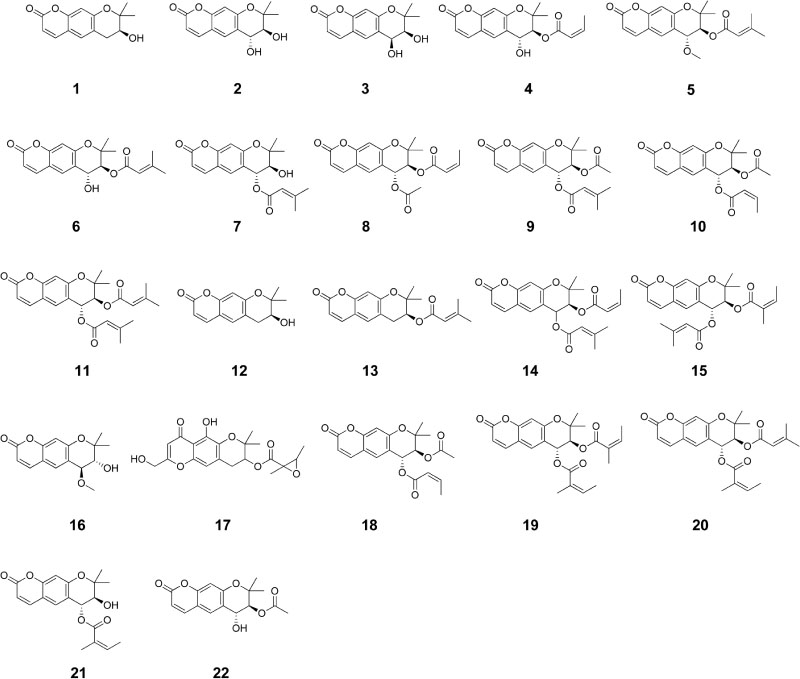
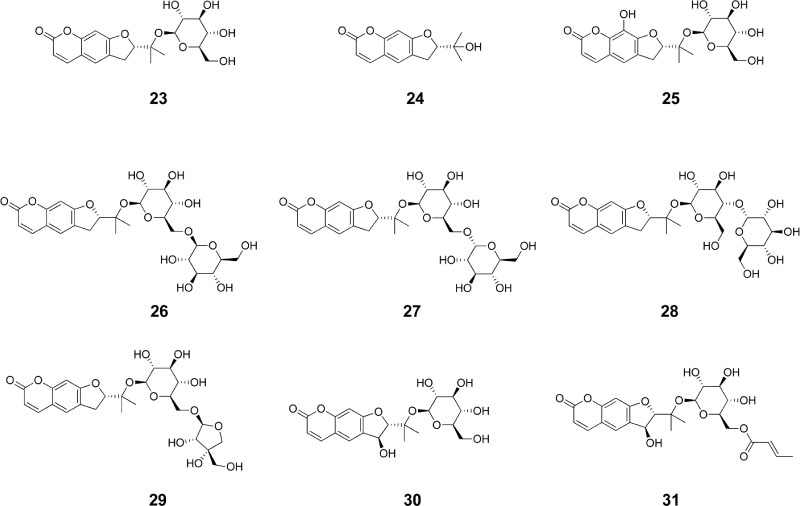
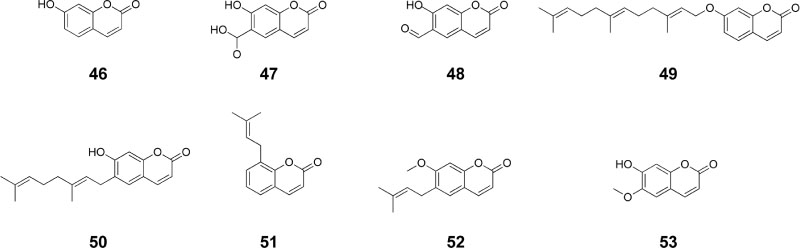
- Angelica decursiva Fr. et Sav. (Umbelliferae) has traditionally been used to treat different diseases due to its antitussive, analgesic, and antipyretic activities. It is also a remedy for thick phlegm, asthma, and upper respiratory infections. Recently, the leaf of A. decursiva has been consumed as salad without showing any toxicity. This plant is a rich in different types of coumarin derivatives, including dihydroxanthyletin, psoralen, dihydropsoralen, hydroxycoumarin, and dihydropyran. Its crude extracts and pure constituents possess anti-inflammatory, anti-diabetic, anti-Alzheimer disease, anti-hypertension, anti-cancer, antioxidant, anthelmintic, preventing cerebral stroke, and neuroprotective activities. This valuable herb needs to be further studied and developed not only to treat these human diseases, but also to improve human health. This review provides an overview of current knowledge of A. decursiva metabolites and their biological activities to prioritize future studies.
Keyword
MeSH Terms
Figure
Reference
-
1. Wei WL, Zeng R, Gu CM, Qu Y, Huang LF. J Ethnopharmacol. 2016; 190:116–141.2. Sowndhararajan K, Deepa P, Kim M, Park SJ, Kim S. Sci Pharm. 2017; 85:33.3. Sarkar SD, Nahar L. Curr Med Chem. 2004; 11:1479–1500.4. Sakakibara I, Okuyama T, Shibata S. Planta Med. 1984; 50:117–120.5. Liu R, Sun Q, Shi Y, Kong L. J Chromatogr A. 2005; 1076:127–132.6. Sarkhail P. J Ethnopharmacol. 2014; 156:235–270.7. Liao ZC, Jiang X, Tian WJ, Lin T, Chen HF. Zhongguo Zhong Yao Za Zhi. 2017; 42:2999–3003.8. Lee SY, Kim CS, Cho SH, Chun HS, Kim JK, Kim DK. J Med Plants Res. 2009; 3:241–245.9. Kong LY, Li Y, Min ZD, Li X, Zhu TR. Phytochemistry. 1996; 41:1423–1426.10. Yi JH, Park IK, Choi KS, Shin SC, Ahn YJ. J Asia Pac Entomol. 2008; 11:221–223.11. Zhao D, Islam MN, Ahn BR, Jung HA, Kim BW, Choi JS. Arch Pharm Res. 2012; 35:179–192.12. Lim HJ, Lee JH, Choi JS, Lee SK, Kim YS, Kim HP. J Ethnopharmacol. 2014; 155:1353–1361.13. Ishita IJ, Nurul Islam M, Kim YS, Choi RJ, Sohn HS, Jung HA, Choi JS. Arch Pharm Res. 2016; 39:115–126.14. Ali MY, Jung HA, Choi JS. Arch Pharm Res. 2015; 38:2216–2227.15. Ali MY, Jannat S, Jung HA, Choi RJ, Roy A, Choi JS. Asian Pac J Trop Med. 2016; 9:103–111.16. Ali MY, Jannat S, Jung HA, Jeong HO, Chung HY, Choi JS. Chem Biol Interact. 2016; 252:93–101.17. Lee AR, Chun JM, Lee AY, Kim HS, Gu GJ, Kwon BN. J Ethnopharmacol. 2017; 196:75–83.18. Ali MY, Seong SH, Reddy MR, Seo SY, Choi JS, Jung HA. Molecules. 2017; 22:1604.19. Ali MY, Jung HA, Jannat S, Choi JS. Arch Pharm Re. 2018; 41:196–207.20. Ali MY, Seong SH, Jung HA, Jannat S, Choi JS. Arch Pharm Res. 2018; 41:753–764.21. Sakakibara I, Okuyama T, Shibata S. Planta Med. 1982; 44:199–203.22. Asahara T, Sakakibara I, Okuyama T, Shibata S. Planta Med. 1984; 50:488–492.23. Xu JF, Kong LY. Zhongguo Zhong Yao Za Zhi. 2001; 26:178–180.24. Chen YC, Chen PY, Wu CC, Tsai IL, Chen IS. J Food Drug Anal. 2008; 16:15–25.25. Jeong SI, Kim JH. Kor Herb Med Inf. 2013; 1:3–24.26. Woo WS, Shin KH, Ryu KS. Arch Pharm Res. 1980; 3:79–84.27. Chi YS, Cheon BS, Kim HP. Biochem Pharmacol. 2001; 61:1195–1203.28. Shu P, Sheh ML. Pollen Photographs and Flora of Umbelliferae in China. Shanghai: Shanghai Scientific and Technical Publisher;2001. p. 68–75.29. Shan RH, Sheh ML. Flora Reipublicae Popularis Sinicae, Tomus 55(3). Beijing: Science Press;1992. p. 123–255.30. Ostroumova TA. Bot Pac. 2018; 7:41.
Article31. Kong LY. J China Pharma Univ. 2010; 41:203–207.32. Asif M. Chem Int. 2015; 1:1–11.33. Xiong Y, Wang J, Yu H, Zhang X, Miao C, Ma S. Immunopharmacol Immunotoxicol. 2014; 36:341–348.34. Islam MN, Choi RJ, Jin SE, Kim YS, Ahn BR, Zhao D, Jung HA, Choi JS. J Ethnopharmacol. 2012; 144:175–181.35. Schlesier K, Harwat M, Böhm V, Bitsch R. Free Radic Res. 2002; 36:177–187.36. Johnson TO, Ermolieff J, Jirousek MR. Nat Rev Drug Discov. 2002; 1:696–709.37. Brownlee M. Nature. 2001; 414:813–820.38. Tomlinson DR, Willars GB, Carrington AL. Pharmacol Ther. 1992; 54:151–194.39. Singh VP, Bali A, Singh N, Jaggi AS. Korean J Physiol Pharmacol. 2014; 18:1–14.40. Cho SH, Kim DK, Kim CS, Lee SY, Chun HS, Kim JK, Jung KY, Lee S, Choi BK. J Korean Soc Appl Biol Chem. 2009; 52:241–246.41. Shin WC, Kim CS, Kim HJ, Lee MH, Kim HR, Kim DK. Int J Oral Biol. 2010; 35:153–158.42. Lee MH, Kim MM, Kook JK, Kim DK, Kim HR, Kim HJ, Kim CS. Int J Oral Biol. 2010; 35:215–220.43. Datta R, Kojima H, Yoshida K, Kufe D. J Biol Chem. 1997; 272:20317.44. Liu X, Zou H, Slaughter C, Wang X. Cell. 1997; 89:175–184.45. Okuyama T, Takata M, Nishino H, Nishino A, Takayasu J, Iwashima A. Chem Pharm Bull. 1990; 38:1084–1086.46. Kang JI, Hong JY, Choi JS, Lee SK. Biomol Ther. 2016; 24:320–327.47. Gao Q, Jeon SJ, Jung HA, Lee HE, Park SJ, Lee Y, Lee Y, Ko SY, Kim B, Choi JS, Ryu JH. Neurochem Res. 2015; 40:1438–1447.48. Wu ZF, Zhu B, Wang Y, Lu C, Wang GX. Parasitol Res. 2011; 108:1557–1563.49. Suzuki T, Kobayashi Y, Uchida MK, Sakakibara I, Okuyama T, ShibataS . J Pharmacobiodyn. 1985; 8:257–263.50. Kim B, Kwon Y, Lee S, Lee K, Ham I, Choi HY. BMC Complement Altern Med. 2017; 17:474.51. Jung SY, Kim KM, Cho S, Lim S, Lim C, Kim YK. Chin Med. 2017; 12:30.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Simultaneous Determination and Recognition Analysis of Coumarins in Angelica decursiva and Peucedanum praeruptorum by HPLC-DAD
- Growth Inhibition of Human Head and Neck Squamous Cell Carcinomas by Angelica decursiva Extracts
- Effects of Nodakenin, Columbianadin, and Umbelliferone Isolated from the Roots of Angelica decursiva on the Gene Expression and Production of MUC5AC Mucin from Human Airway Epithelial NCI-H292 Cells
- Ethanol Extracts of Angelica decursiva Induces Apoptosis in Human Oral Cancer Cells
- Detection of Small Anellovirus DNA from Blood Products