Korean J Ophthalmol.
2019 Oct;33(5):436-445. 10.3341/kjo.2019.0036.
Effects of Orbital Decompression on Lamina Cribrosa Depth in Patients with Graves' Orbitopathy
- Affiliations
-
- 1Department of Ophthalmology, Institute of Vision Research, Severance Hospital, Yonsei University College of Medicine, Seoul, Korea. yoonjs@yuhs.ac
- KMID: 2459525
- DOI: http://doi.org/10.3341/kjo.2019.0036
Abstract
- PURPOSE
We sought to investigate the effects of Graves' orbitopathy (GO) and orbital decompression on lamina cribrosa depth (LCD) using spectral-domain optical coherence tomography.
METHODS
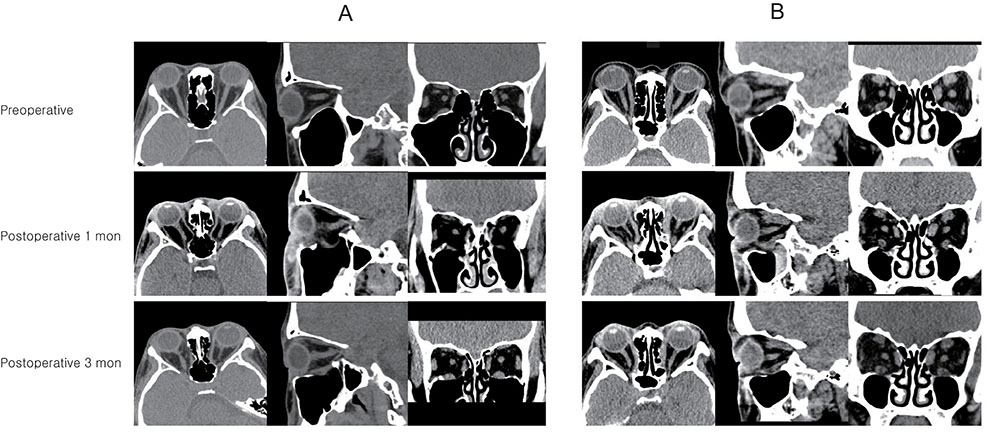
Forty eyes that underwent orbital decompression to relieve compressive optic neuropathy or correct disfiguring exophthalmos in the context of GO were included. Subjects were imaged with spectral-domain optical coherence tomography before surgery and at 1 and 3 months after surgery, at which the examiner measured the LCD (distance from the anterior surface of the lamina cribrosa to the Bruch membrane opening line) and peripapillary retinal nerve fiber layer thickness. Subjects were divided into two groups"”a muscle-dominant group composed of patients who had extraocular muscle enlargement on preoperative orbital computed tomography scan and a fat-dominant group composed of patients who did not show extraocular muscle enlargement on preoperative orbital computed tomography scan"”and subgroup analysis was performed. Preoperative and postoperative intraocular pressure, exophthalmos, LCD, and retinal nerve fiber layer thickness were evaluated.
RESULTS
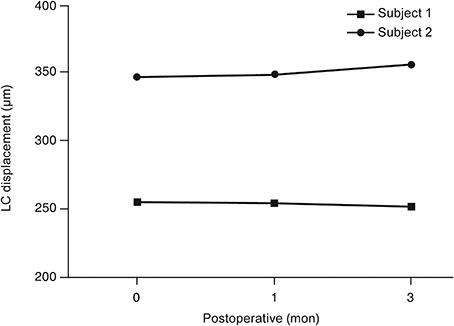
At baseline, LCD was remarkably shallower in the muscle-dominant group than in the fat-dominant group (95% confidence interval, p = 0.007). In the muscle-dominant group, LCD showed no definite change after surgery. However, the fat-dominant group showed temporary posterior displacement of the lamina cribrosa at 1-month postoperation that was reversed to baseline at 3 months postoperation (95% confidence interval, p < 0.01).
CONCLUSIONS
The lamina cribrosa was anteriorly displaced preoperatively, and its position was nearly unchanged after the surgery, especially in association with extraocular muscle enlargement. An enlarged extraocular muscle could reduce the pressure-relieving effect of orbital decompression around the scleral canal in patients with GO.
Keyword
MeSH Terms
Figure
Reference
-
1. Smith TJ, Hegedus L. Graves' disease. N Engl J Med. 2016; 375:1552–1565.
Article2. Dolman PJ. Evaluating Graves' orbitopathy. Best Pract Res Clin Endocrinol Metab. 2012; 26:229–248.
Article3. Garrity JA, Fatourechi V, Bergstralh EJ, et al. Results of transantral orbital decompression in 428 patients with severe Graves' ophthalmopathy. Am J Ophthalmol. 1993; 116:533–547.
Article4. Nik N, Fong A, Derdzakyan M, et al. Changes in choroidal perfusion after orbital decompression surgery for Graves' ophthalmopathy. J Ophthalmic Vis Res. 2017; 12:183–186.5. Burgoyne CF, Downs JC, Bellezza AJ, et al. The optic nerve head as a biomechanical structure: a new paradigm for understanding the role of IOP-related stress and strain in the pathophysiology of glaucomatous optic nerve head damage. Prog Retin Eye Res. 2005; 24:39–73.
Article6. Lee EJ, Kim TW, Weinreb RN. Reversal of lamina cribrosa displacement and thickness after trabeculectomy in glaucoma. Ophthalmology. 2012; 119:1359–1366.
Article7. Burgoyne CF. A biomechanical paradigm for axonal insult within the optic nerve head in aging and glaucoma. Exp Eye Res. 2011; 93:120–132.
Article8. Dev S, Damji KF, DeBacker CM, et al. Decrease in intraocular pressure after orbital decompression for thyroid orbitopathy. Can J Ophthalmol. 1998; 33:314–319.9. Otto AJ, Koornneef L, Mourits MP, Deen-van Leeuwen L. Retrobulbar pressures measured during surgical decompression of the orbit. Br J Ophthalmol. 1996; 80:1042–1045.
Article10. Perez-Lopez M, Sales-Sanz M, Rebolleda G, et al. Retrobulbar ocular blood flow changes after orbital decompression in Graves' ophthalmopathy measured by color Doppler imaging. Invest Ophthalmol Vis Sci. 2011; 52:5612–5617.11. Girard MJ, Tun TA, Husain R, et al. Lamina cribrosa visibility using optical coherence tomography: comparison of devices and effects of image enhancement techniques. Invest Ophthalmol Vis Sci. 2015; 56:865–874.
Article12. Neigel JM, Rootman J, Belkin RI, et al. Dysthyroid optic neuropathy. The crowded orbital apex syndrome. Ophthalmology. 1988; 95:1515–1521.13. Ben Simon GJ, Syed HM, Douglas R, et al. Extraocular muscle enlargement with tendon involvement in thyroid-associated orbitopathy. Am J Ophthalmol. 2004; 137:1145–1147.
Article14. Ozgen A, Ariyurek M. Normative measurements of orbital structures using CT. AJR Am J Roentgenol. 1998; 170:1093–1096.
Article15. Yun SC, Hahn IK, Sung KR, et al. Lamina cribrosa depth according to the level of axial length in normal and glaucomatous eyes. Graefes Arch Clin Exp Ophthalmol. 2015; 253:2247–2253.
Article16. Wilson WB, Manke WF. Orbital decompression in Graves' disease. The predictability of reduction of proptosis. Arch Ophthalmol. 1991; 109:343–345.17. Kennerdell JS, Rosenbaum AE, El-Hoshy MH. Apical optic nerve compression of dysthyroid optic neuropathy on computed tomography. Arch Ophthalmol. 1981; 99:807–809.
Article18. Trokel SL, Jakobiec FA. Correlation of CT scanning and pathologic features of ophthalmic Graves' disease. Ophthalmology. 1981; 88:553–564.
Article19. Kim JW, Lee KH, Woo YJ, et al. The effect of systemic steroids and orbital radiation for active Graves orbitopathy on postdecompression extraocular muscle volume. Am J Ophthalmol. 2016; 171:11–17.
Article20. Motolko M, Drance SM. Features of the optic disc in preglaucomatous eyes. Arch Ophthalmol. 1981; 99:1992–1994.
Article21. Pederson JE, Anderson DR. The mode of progressive disc cupping in ocular hypertension and glaucoma. Arch Ophthalmol. 1980; 98:490–495.
Article22. Sommer A, Pollack I, Maumenee AE. Optic disc parameters and onset of glaucomatous field loss. I. Methods and progressive changes in disc morphology. Arch Ophthalmol. 1979; 97:1444–1448.23. Lee EJ, Kim TW, Kim M, Kim H. Influence of lamina cribrosa thickness and depth on the rate of progressive retinal nerve fiber layer thinning. Ophthalmology. 2015; 122:721–729.
Article24. Lee EJ, Kim TW. Lamina cribrosa reversal after trabeculectomy and the rate of progressive retinal nerve fiber layer thinning. Ophthalmology. 2015; 122:2234–2242.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Orbital Decompression for Dysthyroid Orbitopathy
- Associations between Orbital Morphology and Exophthalmos Changes after Endoscopic Orbital Decompression to Treat Thyroid-related Orbitopathy
- Endoscopic Orbital Decompression for Dysthyroid Orbitopathy
- Lamina Cribrosa Thickness in the Fellow Eyes of Patients with Unilateral Retinal Vein Occlusion
- Evaluation of Stereotactic Navigation During Orbital Decompression in Thyroid-Associated Orbitopathy Patients