J Rheum Dis.
2019 Oct;26(4):257-263. 10.4078/jrd.2019.26.4.257.
YKL-40 Levels in Rheumatoid Arthritis and Their Correlation with Disease Activity: A Meta-analysis
- Affiliations
-
- 1Department of Rheumatology, Korea University College of Medicine, Seoul, Korea. lyhcgh@korea.ac.kr
- KMID: 2459450
- DOI: http://doi.org/10.4078/jrd.2019.26.4.257
Abstract
OBJECTIVE
To examine the relationship of serum/plasma YKL-40 levels with rheumatoid arthritis (RA) and their correlation with RA activity and rheumatoid factor (RF) level.
METHODS
We performed a meta-analysis comparing the serum/plasma YKL-40 levels between patients with RA and controls and examined the correlation coefficients of the circulating YKL-40 level with the RF level and RA activity based on the 28-joint disease activity score (DAS28), erythrocyte sedimentation rate (ESR), and C-reactive protein (CRP) level.
RESULTS
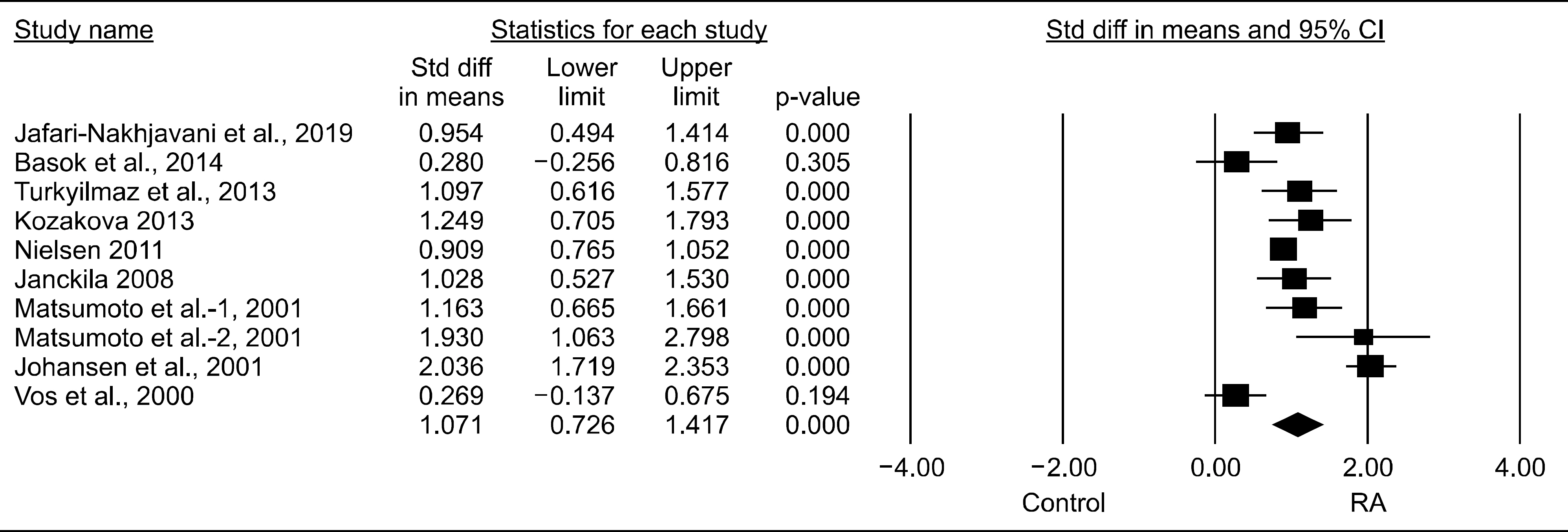
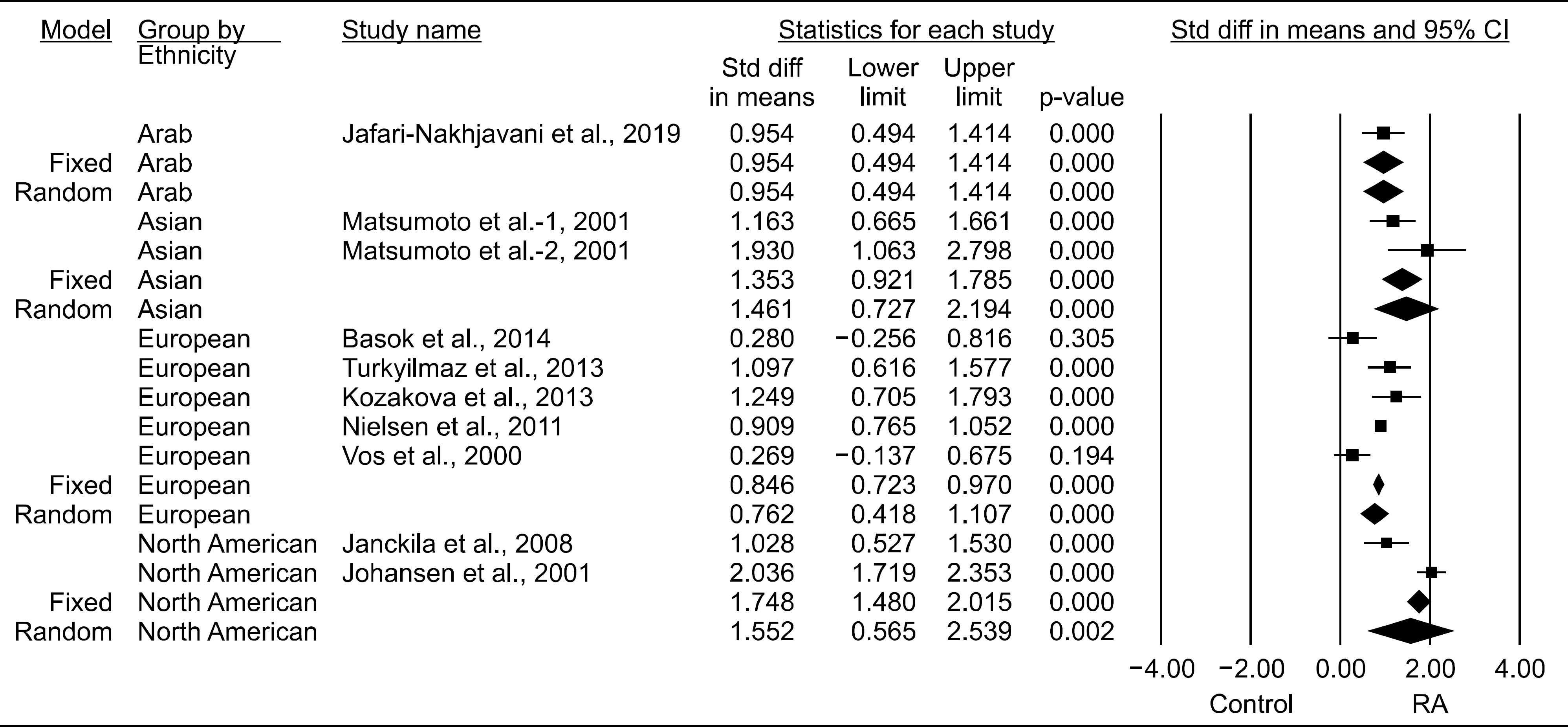
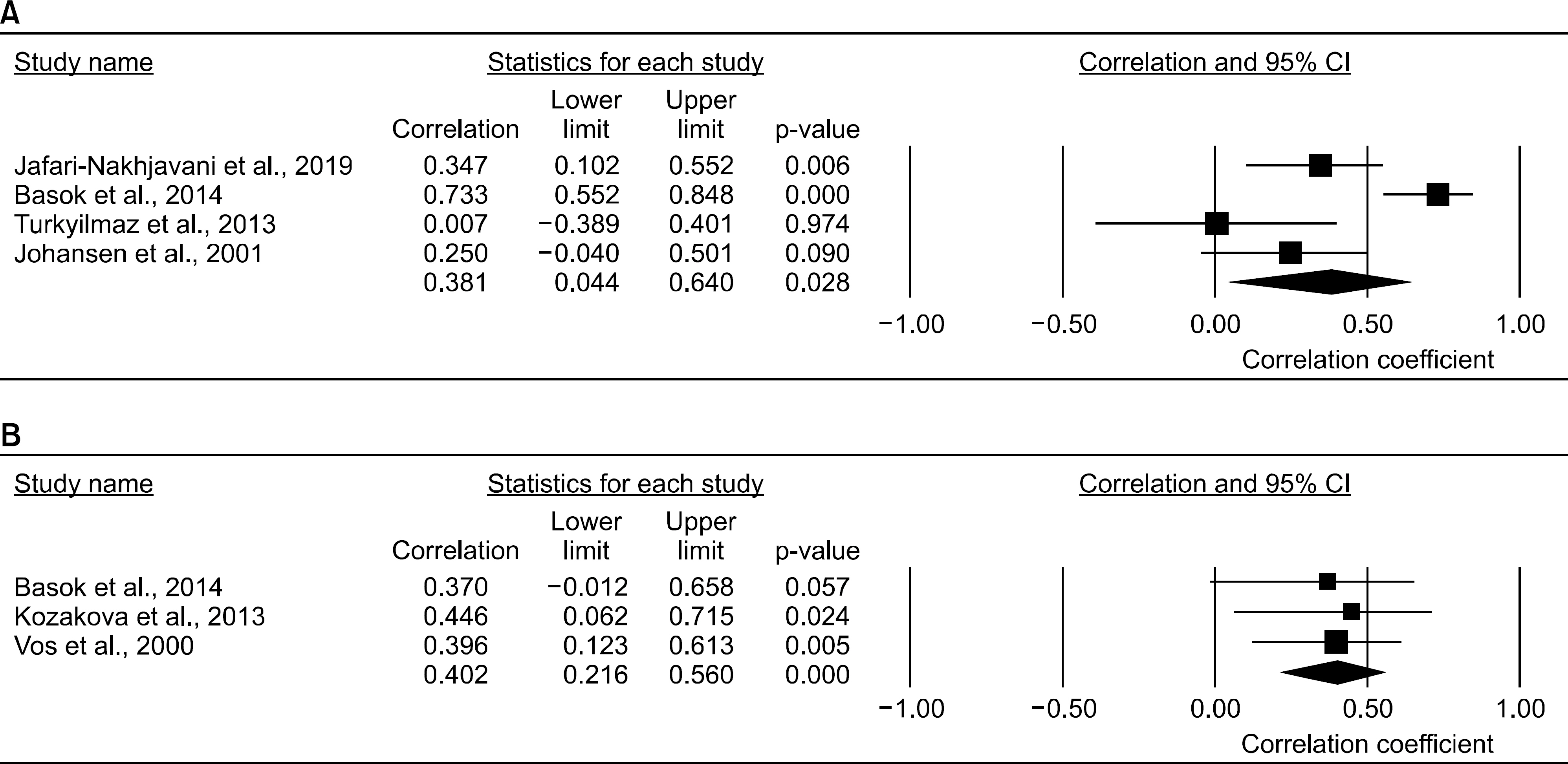
Nine studies (707 patients with RA and 1,041 controls) were included in the meta-analysis. The YKL-40 levels were significantly higher in the RA group than in the control group (standardized mean difference [SMD]=1.071, 95% confidence interval [CI]=0.726~1.417, p<0.001). Stratification by ethnicity showed significantly elevated YKL-40 levels in the RA groups from European, Asian, North American, and Arab populations. The YKL-40 level was significantly higher in the RA group than in the control group in both age- and sex-matched and only age-matched populations (SMD=0.937, 95% CI=0.554~1.320, p<0.001; SMD=2.951, 95% CI=1.389~4.512, p<0.001, respectively). Subgroup analysis by sample size showed significantly increased YKL-40 levels in the RA group in both small (n<100) and large (n>100) populations. Meta-analysis of correlation coefficients showed a significant positive correlation between the YKL-40 levels and DAS28, ESR, CRP level, and RF level (DAS28: correlation coefficient=0.381, 95% CI=0.044~0.640, p=0.028; RF level: correlation coefficient=0.341, 95% CI=0.176~0.487, p<0.001).
CONCLUSION
The circulating YKL-40 levels are high in patients with RA and positively correlate with RA activity and RF level.
Keyword
MeSH Terms
Figure
Reference
-
1. Lee YH, Bae SC, Choi SJ, Ji JD, Song GG. Genomewide pathway analysis of genomewide association studies on systemic lupus erythematosus and rheumatoid arthritis. Mol Biol Rep. 2012; 39:10627–35.
Article2. Hakala BE, White C, Recklies AD. Human cartilage gp-39, a major secretory product of articular chondrocytes and synovial cells, is a mammalian member of a chitinase protein family. J Biol Chem. 1993; 268:25803–10.
Article3. Johansen JS. Studies on serum YKL-40 as a biomarker in diseases with inflammation, tissue remodelling, fibroses and cancer. Dan Med Bull. 2006; 53:172–209.4. Zivanović S, Rackov LP, Vojvodić D, Vucetić D. Human cartilage glycoprotein 39–biomarker of joint damage in knee osteoarthritis. Int Orthop. 2009; 33:1165–70.5. Furuhashi K, Suda T, Nakamura Y, Inui N, Hashimoto D, Miwa S, et al. Increased expression of YKL-40, a chiti-nase-like protein, in serum and lung of patients with idiopathic pulmonary fibrosis. Respir Med. 2010; 104:1204–10.
Article6. Turkyilmaz AK, Devrimsel G, Kirbas A, Cicek Y, Karkucak M, Capkin E, et al. Relationship between pulse wave velocity and serum YKL-40 level in patients with early rheumatoid arthritis. Rheumatol Int. 2013; 33:2751–6.
Article7. Kazakova M, Batalov A, Deneva T, Mateva N, Kolarov Z, Sarafian V. Relationship between sonographic parameters and YKL-40 levels in rheumatoid arthritis. Rheumatol Int. 2013; 33:341–6.
Article8. Nielsen KR, Steffensen R, Boegsted M, Baech J, Lundbye-Christensen S, Hetland ML, et al. Promoter polymorphisms in the chitinase 3-like 1 gene influence the serum concentration of YKL-40 in Danish patients with rheumatoid arthritis and in healthy subjects. Arthritis Res Ther. 2011; 13:R109.
Article9. Johansen JS, Kirwan JR, Price PA, Sharif M. Serum YKL-40 concentrations in patients with early rheumatoid arthritis: relation to joint destruction. Scand J Rheumatol. 2001; 30:297–304.10. Janckila AJ, Neustadt DH, Yam LT. Significance of serum TRACP in rheumatoid arthritis. J Bone Miner Res. 2008; 23:1287–95.
Article11. Vos K, Steenbakkers P, Miltenburg AM, Bos E, van Den Heuvel MW, van Hogezand RA, et al. Raised human cartilage glycoprotein-39 plasma levels in patients with rheumatoid arthritis and other inflammatory conditions. Ann Rheum Dis. 2000; 59:544–8.
Article12. Jafari-Nakhjavani MR, Ghorbanihaghjo A, Bagherzadeh-Nobari B, Malek-Mahdavi A, Rashtchizadeh N. Serum YKL-40 levels and disease characteristics in patients with rheumatoid arthritis. Caspian J Intern Med. 2019; 10:92–7.13. Basok BI, Kucur M, Kizilgul M, Yilmaz I, Ekmekci OB, Uzunlulu M, et al. Increased chitotriosidase activities in patients with rheumatoid arthritis: a possible novel marker? J Med Biochem. 2014; 33:245–51.
Article14. Matsumoto T, Tsurumoto T. Serum YKL-40 levels in rheumatoid arthritis: correlations between clinical and labo-rarory parameters. Clin Exp Rheumatol. 2001; 19:655–60.15. Hozo SP, Djulbegovic B, Hozo I. Estimating the mean and variance from the median, range, and the size of a sample. BMC Med Res Methodol. 2005; 5:13.
Article16. Ridout KK, Ridout SJ, Price LH, Sen S, Tyrka AR. Depression and telomere length: a meta-analysis. J Affect Disord. 2016; 191:237–47.
Article17. Moher D, Liberati A, Tetzlaff J, Altman DG. PRISMA Group. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. PLoS Med. 2009; 6:e1000097.
Article18. McGough JJ, Faraone SV. Estimating the size of treatment effects: moving beyond p values. Psychiatry (Edgmont). 2009; 6:21–9.19. Egger M, Smith GD, Phillips AN. Meta-analysis: principles and procedures. BMJ. 1997; 315:1533–7.
Article20. DerSimonian R, Laird N. Meta-analysis in clinical trials. Control Clin Trials. 1986; 7:177–88.
Article21. Higgins JP, Thompson SG. Quantifying heterogeneity in a meta-analysis. Stat Med. 2002; 21:1539–58.
Article22. Egger M, Davey Smith G, Schneider M, Minder C. Bias in meta-analysis detected by a simple, graphical test. BMJ. 1997; 315:629–34.
Article23. Knudsen LS, Klarlund M, Skjødt H, Jensen T, Ostergaard M, Jensen KE, et al. Biomarkers of inflammation in patients with unclassified polyarthritis and early rheumatoid arthritis. Relationship to disease activity and radiographic outcome. J Rheumatol. 2008; 35:1277–87.24. Peltomaa R, Paimela L, Harvey S, Helve T, Leirisalo-Repo M. Increased level of YKL-40 in sera from patients with early rheumatoid arthritis: a new marker for disease activity. Rheumatol Int. 2001; 20:192–6.
Article25. Ling H, Recklies AD. The chitinase 3-like protein human cartilage glycoprotein 39 inhibits cellular responses to the inflammatory cytokines interleukin-1 and tumour necrosis factor-alpha. Biochem J. 2004; 380:651–9.
Article26. Recklies AD, Ling H, White C, Bernier SM. Inflammatory cytokines induce production of CHI3L1 by articular chondrocytes. J Biol Chem. 2005; 280:41213–21.
Article27. Lee CG, Da Silva CA, Dela Cruz CS, Ahangari F, Ma B, Kang MJ, et al. Role of chitin and chitinase/chitinase-like proteins in inflammation, tissue remodeling, and injury. Annu Rev Physiol. 2011; 73:479–501.
Article28. Joosten LA, Coenen-de Roo CJ, Helsen MM, Lubberts E, Boots AM, van den Berg WB, et al. Induction of tolerance with intranasal administration of human cartilage gp-39 in DBA/1 mice: amelioration of clinical, histologic, and radiologic signs of type II collagen-induced arthritis. Arthritis Rheum. 2000; 43:645–55.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Circulating leptin and its correlation with rheumatoid arthritis activity: a meta-analysis
- Serum Interleukin-10 Levels in Rheumatoid Arthritis Patients
- Circulating Interleukin-37 Levels in Rheumatoid Arthritis and Systemic Lupus Erythematosus and Their Correlations With Disease Activity: A Meta-analysis
- Utility of Serum YKL-40 as a Tumor-Specific Marker of Hepatobiliary Malignancies
- A Study on the Relationship between Mastery and Activity of Daily Life in Rheumatoid Arthritis Patients