Korean J Radiol.
2019 Nov;20(11):1536-1545. 10.3348/kjr.2019.0104.
Altered Functional Brain Networks in Patients with Traumatic Anosmia: Resting-State Functional MRI Based on Graph Theoretical Analysis
- Affiliations
-
- 1Department of Radiology, Konkuk University Medical Center, Konkuk University School of Medicine, Seoul, Korea. mdmoonwj@naver.com
- 2Department of Radiology, Gangnam Severance Hospital, Yonsei University College of Medicine, Seoul, Korea.
- 3Laboratory for Cognitive Neuroscience and NeuroImaging, Department of Bio and Brain Engineering, and KI for Health Science and Technology, Korea Advanced Institute of Science and Technology (KAIST), Daejeon, Korea. yong@kaist.ac.kr
- 4Department of Otorhinolaryngology-Head and Neck Surgery, Konkuk University Medical Center, Konkuk University School of Medicine, Seoul, Korea.
- KMID: 2459421
- DOI: http://doi.org/10.3348/kjr.2019.0104
Abstract
OBJECTIVE
Traumatic anosmia is a common disorder following head injury; however, little is known regarding its neural basis and influence on the functional networks. Therefore, we aimed to investigate the functional connectivity changes in patients with traumatic anosmia compared to healthy controls using resting-state functional magnetic resonance imaging (rs-fMRI).
MATERIALS AND METHODS
Sixteen patients with traumatic anosmia and 12 healthy controls underwent rs-fMRI. Differences in the connectivity of the olfactory and whole brain networks were compared between the two groups. Graph theoretical parameters, such as modularity and global efficiency of the whole brain or olfactory networks, were calculated and compared. Correlation analyses were performed between the parameters and disease severity.
RESULTS
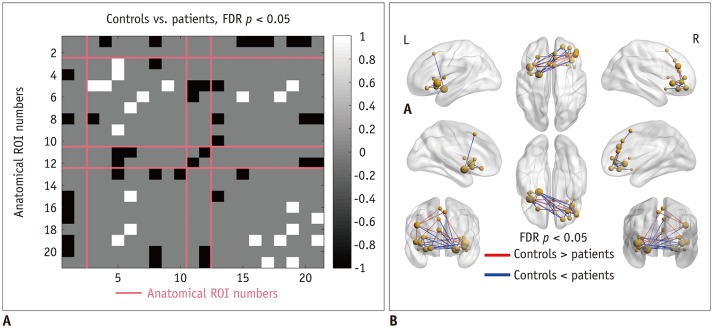
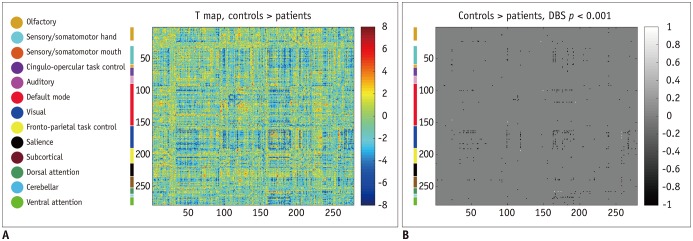
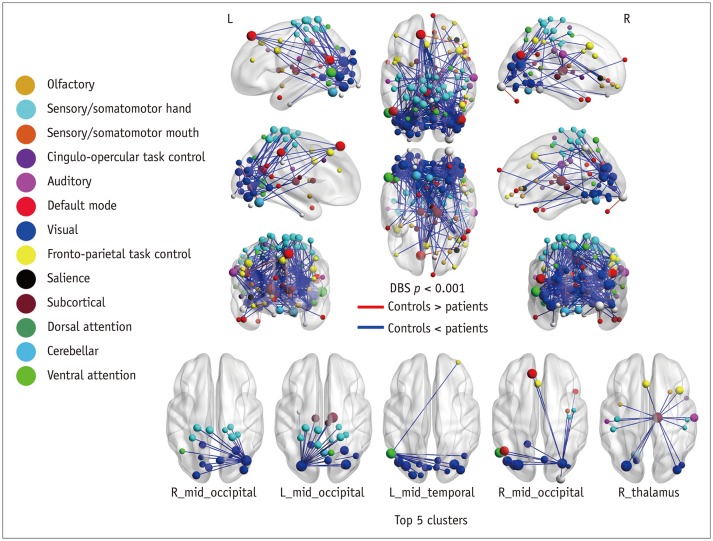
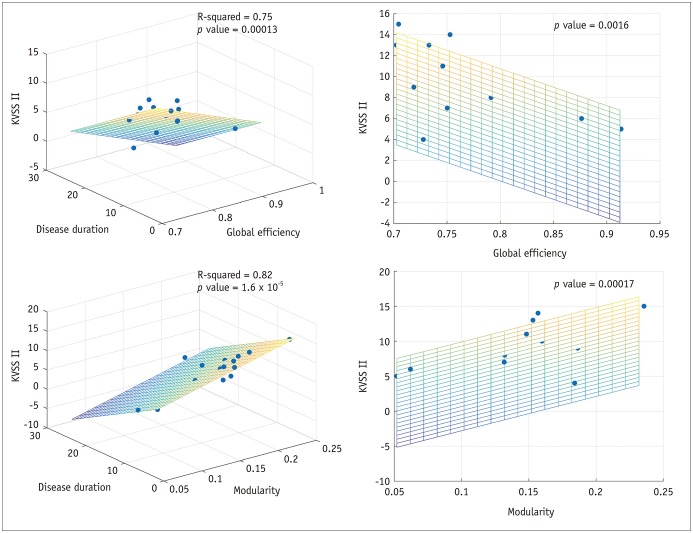
Patients with traumatic anosmia showed decreased intra-network connectivity in the olfactory network (false discovery rate [FDR]-corrected p < 0.05) compared with that in healthy controls. Furthermore, the inter-network connectivity was increased in both the olfactory (FDR-corrected p < 0.05) and whole brain networks (degree-based statistic-corrected p < 0.05) in the anosmia group. The whole brain networks showed decreased modularity (p < 0.001) and increased global efficiency (p = 0.019) in patients with traumatic anosmia. The modularity and global efficiency were correlated with disease severity in patients with anosmia (p < 0.001 and p = 0.002, respectively).
CONCLUSION
Traumatic anosmia increased the inter-network connectivity observed with rs-fMRI in the olfactory and global brain functional networks. rs-fMRI parameters may serve as potential biomarkers for traumatic anosmia by revealing a more widespread functional damage than previously expected.
Keyword
MeSH Terms
Figure
Reference
-
1. Costanzo RM, Miwa T. Posttraumatic olfactory loss. Adv Otorhinolaryngol. 2006; 63:99–107. PMID: 16733335.
Article2. Mott AE, Leopold DA. Disorders in taste and smell. Med Clin North Am. 1991; 75:1321–1353. PMID: 1943323.
Article3. Schofield PW, Moore TM, Gardner A. Traumatic brain injury and olfaction: a systematic review. Front Neurol. 2014; 5:5. PMID: 24478752.
Article4. Miao X, Yang L, Gu H, Ren Y, Chen G, Liu J, et al. Evaluation of post-traumatic anosmia with MRI and chemosensory ERPs. Eur Arch Otorhinolaryngol. 2015; 272:1945–1953. PMID: 25253545.
Article5. Yousem DM, Geckle RJ, Bilker WB, McKeown DA, Doty RL. Posttraumatic olfactory dysfunction: MR and clinical evaluation. AJNR Am J Neuroradiol. 1996; 17:1171–1179. PMID: 8791933.6. Henkin RI, Levy LM. Functional MRI of congenital hyposmia: brain activation to odors and imagination of odors and tastes. J Comput Assist Tomogr. 2002; 26:39–61. PMID: 11801904.
Article7. Kollndorfer K, Fischmeister FP, Kowalczyk K, Hoche E, Mueller CA, Trattnig S, et al. Olfactory training induces changes in regional functional connectivity in patients with long-term smell loss. Neuroimage Clin. 2015; 9:401–410. PMID: 26594622.
Article8. Moon WJ, Park M, Hwang M, Kim JK. Functional MRI as an objective measure of olfaction deficit in patients with traumatic anosmia. AJNR Am J Neuroradiol. 2018; 39:2320–2325. PMID: 30409849.
Article9. Reichert JL, Postma EM, Smeets PAM, Boek WM, de Graaf K, Schöpf V, et al. Severity of olfactory deficits is reflected in functional brain networks-An fMRI study. Hum Brain Mapp. 2018; 39:3166–3177. PMID: 29602198.
Article10. Hummel T, Fliessbach K, Abele M, Okulla T, Reden J, Reichmann H, et al. Olfactory FMRI in patients with Parkinson's disease. Front Integr Neurosci. 2010; 4:125. PMID: 21120143.
Article11. Schneider F, Habel U, Reske M, Toni I, Falkai P, Shah NJ. Neural substrates of olfactory processing in schizophrenia patients and their healthy relatives. Psychiatry Res. 2007; 155:103–112. PMID: 17532193.
Article12. Wang J, Eslinger PJ, Doty RL, Zimmerman EK, Grunfeld R, Sun X, et al. Olfactory deficit detected by fMRI in early Alzheimer's disease. Brain Res. 2010; 1357:184–194. PMID: 20709038.
Article13. Vedaei F, Fakhri M, Harirchian MH, Firouznia K, Lotfi Y, Ali Oghabian M. Methodological considerations in conducting an olfactory fMRI study. Behav Neurol. 2013; 27:267–276. PMID: 23619085.
Article14. Hohenfeld C, Werner CJ, Reetz K. Resting-state connectivity in neurodegenerative disorders: is there potential for an imaging biomarker? Neuroimage Clin. 2018; 18:849–870. PMID: 29876270.
Article15. Kollndorfer K, Jakab A, Mueller CA, Trattnig S, Schöpf V. Effects of chronic peripheral olfactory loss on functional brain networks. Neuroscience. 2015; 310:589–599. PMID: 26415766.
Article16. Hong SC, Yoo YS, Kim ES, Kim SC, Park SH, Kim JK, et al. Development of KVSS test (Korean version of Sniffin' Sticks test). Korean J Otolaryngol-Head Neck Surg. 1999; 42:855–860.17. Cho JH, Jeong YS, Lee YJ, Hong SC, Yoon JH, Kim JK. The Korean version of the Sniffin' stick (KVSS) test and its validity in comparison with the cross-cultural smell identification test (CC-SIT). Auris Nasus Larynx. 2009; 36:280–286. PMID: 18775610.
Article18. Friston K, Harrison L, Daunizeau J, Kiebel S, Phillips C, Trujillo-Barreto N, et al. Multiple sparse priors for the M/EEG inverse problem. Neuroimage. 2008; 39:1104–1120. PMID: 17997111.
Article19. Collignon A, Maes F, Delaere D, Vandermeulen D, Suetens P, Marchal G. Automated multi-modality image registration based on information theory. In : Bizais Y, Barillot C, Di Paola R, editors. Information processing in medical imaging. Dordrecht: Kluwer Academic Publishers;1995. p. 263–274.20. Rao H, Korczykowski M, Pluta J, Hoang A, Detre JA. Neural correlates of voluntary and involuntary risk taking in the human brain: an fMRI study of the Balloon Analog Risk Task (BART). Neuroimage. 2008; 42:902–910. PMID: 18582578.
Article21. Shah C, Erhard K, Ortheil HJ, Kaza E, Kessler C, Lotze M. Neural correlates of creative writing: an fMRI study. Hum Brain Mapp. 2013; 34:1088–1101. PMID: 22162145.
Article22. Power JD, Cohen AL, Nelson SM, Wig GS, Barnes KA, Church JA, et al. Functional network organization of the human brain. Neuron. 2011; 72:665–678. PMID: 22099467.
Article23. Seubert J, Freiherr J, Djordjevic J, Lundström JN. Statistical localization of human olfactory cortex. Neuroimage. 2013; 66:333–342. PMID: 23103688.
Article24. Pamplona GS, Santos Neto GS, Rosset SR, Rogers BP, Salmon CE. Analyzing the association between functional connectivity of the brain and intellectual performance. Front Hum Neurosci. 2015; 9:61. PMID: 25713528.
Article25. Rubinov M, Sporns O. Complex network measures of brain connectivity: uses and interpretations. Neuroimage. 2010; 52:1059–1069. PMID: 19819337.
Article26. Latora V, Marchiori M. Efficient behavior of small-world networks. Phys Rev Lett. 2001; 87:198701. PMID: 11690461.
Article27. Blondel VD, Guillaume JL, Lambiotte R, Lefebvre E. Fast unfolding of communities in large networks. J Stat Mech. 2008; 2008:P10008.
Article28. Chung J, Yoo K, Lee P, Kim CM, Roh JH, Park JE, et al. Normalization of cortical thickness measurements across different T1 magnetic resonance imaging protocols by novel W-score standardization. Neuroimage. 2017; 159:224–235. PMID: 28757193.
Article29. Groppe DM, Urbach TP, Kutas M. Mass univariate analysis of event-related brain potentials/fields I: a critical tutorial review. Psychophysiology. 2011; 48:1711–1725. PMID: 21895683.
Article30. Benjamini Y, Hochberg Y. Controlling the false discovery rate: a practical and powerful approach to multiple testing. J R Stat Soc B. 1995; 57:289–300.
Article31. Yoo K, Lee P, Chung MK, Sohn WS, Chung SJ, Na DL, et al. Degree-based statistic and center persistency for brain connectivity analysis. Hum Brain Mapp. 2017; 38:165–181. PMID: 27593391.
Article32. Han P, Winkler N, Hummel C, Hähner A, Gerber J, Hummel T. Alterations of brain gray matter density and olfactory bulb volume in patients with olfactory loss after traumatic brain injury. J Neurotrauma. 2018; 35:2632–2640. PMID: 29699465.
Article33. Damoiseaux JS, Prater KE, Miller BL, Greicius MD. Functional connectivity tracks clinical deterioration in Alzheimer's disease. Neurobiol Aging. 2012; 33:828.e19–828.e30.
Article34. Bettus G, Guedj E, Joyeux F, Confort-Gouny S, Soulier E, Laguitton V, et al. Decreased basal fMRI functional connectivity in epileptogenic networks and contralateral compensatory mechanisms. Hum Brain Mapp. 2009; 30:1580–1591. PMID: 18661506.
Article35. Kollndorfer K, Kowalczyk K, Hoche E, Mueller CA, Pollak M, Trattnig S, et al. Recovery of olfactory function induces neuroplasticity effects in patients with smell loss. Neural Plast. 2014; 2014:140419. PMID: 25544900.
Article36. Bende M, Nordin S. Perceptual learning in olfaction: professional wine tasters versus controls. Physiol Behav. 1997; 62:1065–1070. PMID: 9333201.37. van den Heuvel MP, Hulshoff Pol HE. Exploring the brain network: a review on resting-state fMRI functional connectivity. Eur Neuropsychopharmacol. 2010; 20:519–534. PMID: 20471808.
Article38. Stanley ML, Simpson SL, Dagenbach D, Lyday RG, Burdette JH, Laurienti PJ. Changes in brain network efficiency and working memory performance in aging. PLoS One. 2015; 10:e0123950. PMID: 25875001.
Article39. Meunier D, Achard S, Morcom A, Bullmore E. Age-related changes in modular organization of human brain functional networks. Neuroimage. 2009; 44:715–723. PMID: 19027073.
Article40. Song J, Birn RM, Boly M, Meier TB, Nair VA, Meyerand ME, et al. Age-related reorganizational changes in modularity and functional connectivity of human brain networks. Brain Connect. 2014; 4:662–676. PMID: 25183440.
Article41. Alexander-Bloch AF, Gogtay N, Meunier D, Birn R, Clasen L, Lalonde F, et al. Disrupted modularity and local connectivity of brain functional networks in childhood-onset schizophrenia. Front Syst Neurosci. 2010; 4:147. PMID: 21031030.
Article42. Brier MR, Thomas JB, Fagan AM, Hassenstab J, Holtzman DM, Benzinger TL, et al. Functional connectivity and graph theory in preclinical Alzheimer's disease. Neurobiol Aging. 2014; 35:757–768. PMID: 24216223.
Article43. Baum GL, Ciric R, Roalf DR, Betzel RF, Moore TM, Shinohara RT, et al. Modular segregation of structural brain networks supports the development of executive function in youth. Curr Biol. 2017; 27:1561–1572.e8. PMID: 28552358.
Article44. Sporns O. The non-random brain: efficiency, economy, and complex dynamics. Front Comput Neurosci. 2011; 5:5. PMID: 21369354.
Article45. Nakayama N, Okumura A, Shinoda J, Yasokawa YT, Miwa K, Yoshimura SI, et al. Evidence for white matter disruption in traumatic brain injury without macroscopic lesions. J Neurol Neurosurg Psychiatry. 2006; 77:850–855. PMID: 16574734.
Article46. Eierud C, Craddock RC, Fletcher S, Aulakh M, King-Casas B, Kuehl D, et al. Neuroimaging after mild traumatic brain injury: review and meta-analysis. Neuroimage Clin. 2014; 4:283–294. PMID: 25061565.
Article47. Akiki TJ, Averill CL, Wrocklage KM, Scott JC, Averill LA, Schweinsburg B, et al. Default mode network abnormalities in posttraumatic stress disorder: a novel network-restricted topology approach. Neuroimage. 2018; 176:489–498. PMID: 29730491.
Article48. Zhou Y. Small world properties changes in mild traumatic brain injury. J Magn Reson Imaging. 2017; 46:518–527. PMID: 27902865.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Variations of Resting-State EEG-Based Functional Networks in Brain Maturation From Early Childhood to Adolescence
- Resting-State Electroencephalography (EEG) Functional Connectivity Analysis
- Change of Brain Functional Connectivity in Patients With Spinal Cord Injury: Graph Theory Based Approach
- Disrupted Control Network Connectivity in Abstinent Patients with Alcohol Dependence
- Cortical Thickness of Resting State Networks in the Brain of Male Patients with Alcohol Dependence