Lab Anim Res.
2018 Dec;34(4):288-294. 10.5625/lar.2018.34.4.288.
Inhibition of endoplasmic reticulum stress in high-fat-diet-induced obese C57BL/6 mice: Efficacy of a novel extract from mulberry (Morus alba) leaves fermented with Cordyceps militaris
- Affiliations
-
- 1Department of Biomaterials Science, College of Natural Resources & Life Science/Life and Industry Convergence Research Institute, Pusan National University, Miryang, Korea. dyhwang@pusan.ac.kr
- 2Department of Horticultural Bioscience, College of Natural Resources & Life Science, Pusan National University, Miryang, Korea.
- 3Novarex Co., Chungju, Korea.
- KMID: 2459307
- DOI: http://doi.org/10.5625/lar.2018.34.4.288
Abstract
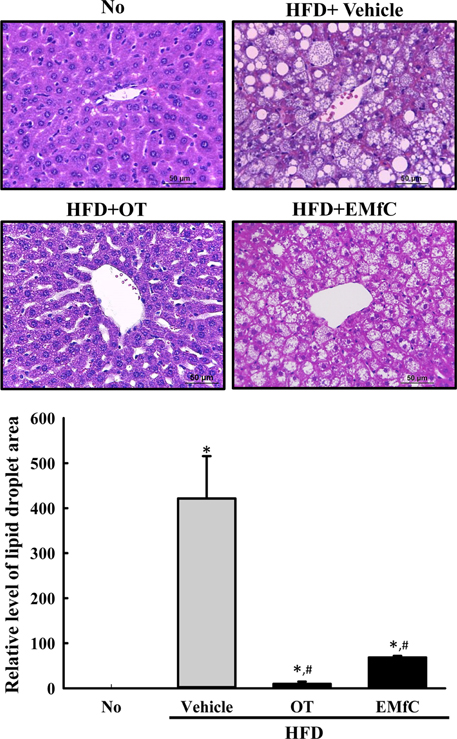
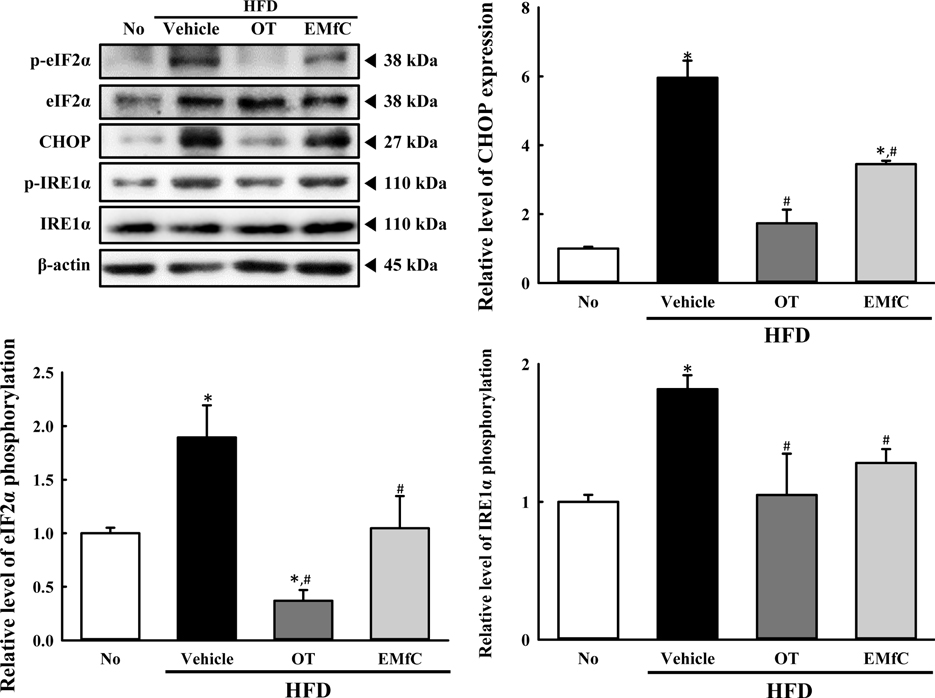
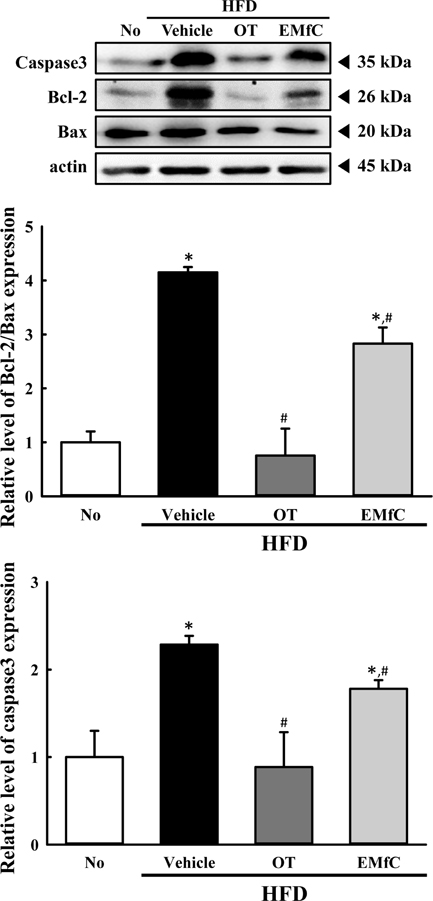
- A few clues about correlation between endoplasmic reticulum (ER) stress and mulberry (Morus alba) leaves were investigated in only the experimental autoimmune myocarditis and streptozotocin-induced diabetes. To investigate whether a novel extract of mulberry leaves fermented with Cordyceps militaris (EMfC) could suppress ER in fatty liver, alterations in the key parameters for ER stress response were measured in high fat diet (HFD)-induced obese C57L/6 mice treated with EMfC for 12 weeks. The area of adipocytes in the liver section were significantly decreased in the HFD+EMfC treated group as compared to the HFD+Vehicle treated group, while their level was higher in HFD+Vehicle treated group than No treated group. The level of the eukaryotic initiation factor 2 alpha (eIF2α) and inositol-requiring enzyme 1 beta (IRE1α) phosphorylation and CCAAT-enhancer-binding protein homologous protein (CHOP) expression were remarkably enhanced in the HFD+Vehicle treated group. However, their levels were restored in the HFD+EMfC treated group, although some differences were detected in the decrease rate. Similar recovery was observed on the ER stress-induced apoptosis. The level of Caspase-3, Bcl-2 and Bax were decreased in the HFD+EMfC and HFD+orlistat (OT) treated group compared to the HFD+Vehicle treated group. The results of the present study therefore provide first evidence that EMfC with the anti-obesity effects can be suppressed ER stress and ER stress-induced apoptosis in the hepatic steatosis of HFD-induced obesity model.
Keyword
MeSH Terms
-
Adipocytes
Animals
Apoptosis
Caspase 3
CCAAT-Enhancer-Binding Proteins
Cordyceps*
Diet, High-Fat
Endoplasmic Reticulum Stress*
Endoplasmic Reticulum*
Eukaryotic Initiation Factor-2
Fatty Liver
Liver
Mice*
Morus*
Myocarditis
Obesity
Phosphorylation
CCAAT-Enhancer-Binding Proteins
Caspase 3
Eukaryotic Initiation Factor-2
Figure
Reference
-
1. Ron D, Walter P. Signal integration in the endoplasmic reticulum unfolded protein response. Nat Rev Mol Cell Biol. 2007; 8(7):519–529.
Article2. Kars M, Yang L, Gregor MF, Mohammed BS, Pietka TA, Finck BN, Patterson BW, Horton JD, Mittendorfer B, Hotamisligil GS, Klein S. Tauroursodeoxycholic Acid may improve liver and muscle but not adipose tissue insulin sensitivity in obese men and women. Diabetes. 2010; 59(8):1899–1905.
Article3. Yoshida H. ER stress and diseases. FEBS J. 2007; 274(3):630–658.
Article4. Forman MS, Lee VM, Trojanowski JQ. ‘Unfolding’ pathways in neurodegenerative disease. Trends Neurosci. 2003; 26(8):407–410.
Article5. Ozcan L, Tabas I. Role of endoplasmic reticulum stress in metabolic disease and other disorders. Annu Rev Med. 2012; 63:317–328.
Article6. Kim JE, Go J, Sung JE, Lee HA, Seo EJ, Yun WB, Hwang DY. Laxative effects of Liriope platyphylla are tightly correlated with suppression of endoplasmic reticulum stress in loperamide-induced constipation of SD rats. Lab Anim Res. 2016; 32(1):16–23.7. Achard CS, Laybutt DR. Lipid-induced endoplasmic reticulum stress in liver cells results in two distinct outcomes: adaptation with enhanced insulin signaling or insulin resistance. Endocrinology. 2012; 153(5):2164–2177.
Article8. Jeong JW, Lee B, Kim DH, Jeong HO, Moon KM, Kim MJ, Yokozawa T, Chung HY. Mechanism of action of magnesium lithospermate B against aging and obesity-induces ER stress, insulin resistance, and inflammsome formation in the liver. Molecules. 2018; 23(9):E2098.9. Kim HM, Kim Y, Lee ES, Huh JH, Chung CH. Caffeic acid ameliorates hepatic steatosis and reduces ER stress in high fat diet-induced obese mice by regulating autophagy. Nutrition. 2018; 55-56:63–70.
Article10. Chen Y, Wu Z, Zhao S, Xiang R. Chemical chaperones reduce ER stress and adipose tissue inflammation in high fat diet-induced mouse model of obesity. Sci Rep. 2016; 6:27486.
Article11. Seoane-Collazo P, Martínez de Morentin PB, Fernø J, Diéguez C, Nogueiras R, López M. Nicotine improves obesity and hepatic steatosis and ER stress in diet-induced obese male rats. Endocrinology. 2014; 155(5):1679–1689.
Article12. Arumugam S, Thandavarayan RA, Veeraveedu PT, Ma M, Giridharan VV, Arozal W, Sari FR, Sukumaran V, Lakshmanan A, Soetikno V, Suzuki K, Kodama M, Watanabe K. Modulation of endoplasmic reticulum stress and cardiomyocyte apoptosis by mulberry leaf diet in experimental autoimmune myocarditis rats. J Clin Biochem Nutr. 2012; 50(2):139–144.
Article13. Arumugam S, Mito S, Thandavarayan RA, Giridharan VV, Pitchaimani V, Karuppagounder V, Harima M, Nomoto M, Suzuki K, Watanabe K. Mulberry leaf diet protects against progression of experimental autoimmune myocarditis to dilated cardiomyopathy via modulation of oxidative stress and MAPK-mediated apoptosis. Cardiovasc Ther. 2013; 31(6):352–362.
Article14. Lee MR, Kim JE, Yun WB, Choi JY, Park JJ, Kim HR, Song BR, Choi YW, Kim KM, Hwang DY. Lipolytic effect of novel extracts from mulberry (Morus alba) leaves fermented with Cordyceps militaris in the primary adipocytes derived from SD rats. Lab Anim Res. 2017; 33(3):270–279.15. Scull CM, Tabas I. Mechanisms of ER stress-induced apoptosis in atherosclerosis. Arterioscler Thromb Vasc Biol. 2011; 31(12):2792–2797.
Article16. Ozcan U, Cao Q, Yilmaz E, Lee AH, Iwakoshi NN, Ozdelen E, Tuncman G, Görgün C, Glimcher LH, Hotamisligil GS. Endoplasmic reticulum stress links obesity, insulin action, and type 2 diabetes. Science. 2004; 306(5695):457–461.
Article17. Puri P, Mirshahi F, Cheung O, Natarajan R, Maher JW, Kellum JM, Sanyal AJ. Activation and dysregulation of the unfolded protein response in nonalcoholic fatty liver disease. Gastroenterology. 2008; 134(2):568–576.
Article18. Cnop M, Foufelle F, Velloso LA. Endoplasmic reticulum stress, obesity and diabetes. Trends Mol Med. 2012; 18(1):59–68.
Article19. Oyadomari S, Harding HP, Zhang Y, Oyadomari M, Ron D. Dephosphorylation of translation initiation factor 2alpha enhances glucose tolerance and attenuates hepatosteatosis in mice. Cell Metab. 2008; 7(6):520–532.20. Kammoun HL, Chabanon H, Hainault I, Luquet S, Magnan C, Koike T, Ferré P, Foufelle F. GRP78 expression inhibits insulin and ER stress-induced SREBP-1c activation and reduces hepatic steatosis in mice. J Clin Invest. 2009; 119(5):1201–1215.
Article21. Heck AM, Yanovski JA, Calis KA. Orlistat, a new lipase inhibitor for the management of obesity. Pharmacotherapy. 2000; 20(3):270–279.
Article22. Zavoral JH. Treatment with orlistat reduces cardiovascular risk in obese patients. J Hypertens. 1998; 16(12 Pt 2):2013–2017.
Article23. Hollander PA, Elbein SC, Hirsch IB, Kelley D, McGill J, Taylor T, Weiss SR, Crockett SE, Kaplan RA, Comstock J, Lucas CP, Lodewick PA, Canovatchel W, Chung J, Hauptman J. Role of orlistat in the treatment of obese patients with type 2 diabetes. A 1-year randomized double-blind study. Diabetes Care. 1998; 21(8):1288–1294.
Article24. Little JL, Wheeler FB, Fels DR, Koumenis C, Kridel SJ. Inhibition of fatty acid synthase induces endoplasmic reticulum stress in tumor cells. Cancer Res. 2007; 67(3):1262–1269.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Lipolytic effect of novel extracts from mulberry (Morus alba) leaves fermented with Cordyceps militaris in the primary adipocytes derived from SD rats
- Effect of combined mulberry leaf and fruit extract on liver and skin cholesterol transporters in high fat diet-induced obese mice
- Effects of quercetin derivatives from mulberry leaves: Improved gene expression related hepatic lipid and glucose metabolism in short-term high-fat fed mice
- Effects of fermented blueberry liquid in high-fat diet-induced obese C57BL/6J mice
- Effects of intermittent ladder-climbing exercise training on mitochondrial biogenesis and endoplasmic reticulum stress of the cardiac muscle in obese middle-aged rats