Lab Anim Res.
2018 Dec;34(4):195-202. 10.5625/lar.2018.34.4.195.
Hyperglycemia aggravates decrease in alpha-synuclein expression in a middle cerebral artery occlusion model
- Affiliations
-
- 1Department of Anatomy, College of Veterinary Medicine, Research Institute of Life Science, Gyeongsang National University, Jinju, Korea. pokoh@gnu.ac.kr
- 2Division of Life Science and Applied Life Science, College of Natural Sciences, Gyeongsang National University, Jinju, Korea.
- 3Department of Endocrine Surgery, Gyeongsang National University School of Medicine and Gyeongsang National University Changwon Hospital, Changwon, Korea.
- KMID: 2459295
- DOI: http://doi.org/10.5625/lar.2018.34.4.195
Abstract
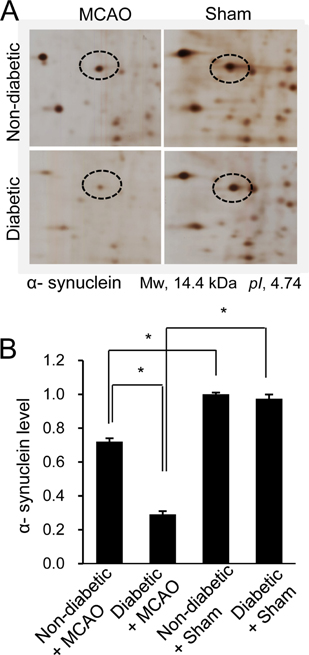
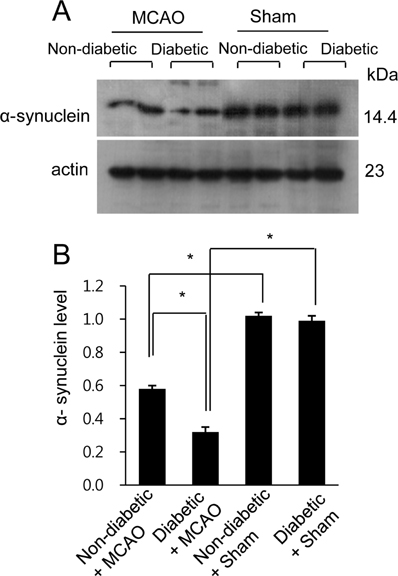
- Hyperglycemia is one of the major risk factors for stroke. Hyperglycemia can lead to a more extensive infarct volume, aggravate neuronal damage after cerebral ischemia. α-Synuclein is especially abundant in neuronal tissue, where it underlies the etiopathology of several neurodegenerative diseases. This study investigated whether hyperglycemic conditions regulate the expression of α-synuclein in middle cerebral artery occlusion (MCAO)-induced cerebral ischemic injury. Male Sprague-Dawley rats were treated with streptozotocin (40 mg/kg) via intraperitoneal injection to induce hyperglycemic conditions. MCAO were performed four weeks after streptozotocin injection to induce focal cerebral ischemia, and cerebral cortex tissues were obtained 24 hours after MCAO. We confirmed that MCAO induced neurological functional deficits and cerebral infarction, and these changes were more extensive in diabetic animals compared to non-diabetic animals. Moreover, we identified a decrease in α-synuclein after MCAO injury. Diabetic animals showed a more serious decrease in α-synuclein than non-diabetic animals. Western blot and reverse-transcription PCR analyses confirmed more extensive decreases in α-synuclein expression in MCAO-injured animals with diabetic condition than these of non-diabetic animals. It is accepted that α-synuclein modulates neuronal cell death and exerts a neuroprotective effect. Thus, the results of this study suggest that hyperglycemic conditions cause more serious brain damage in ischemic brain injuries by decreasing α-synuclein expression.
Keyword
MeSH Terms
-
alpha-Synuclein*
Animals
Blotting, Western
Brain
Brain Injuries
Brain Ischemia
Cell Death
Cerebral Cortex
Cerebral Infarction
Humans
Hyperglycemia*
Infarction, Middle Cerebral Artery*
Injections, Intraperitoneal
Male
Middle Cerebral Artery*
Neurodegenerative Diseases
Neurons
Neuroprotective Agents
Polymerase Chain Reaction
Rats, Sprague-Dawley
Risk Factors
Streptozocin
Stroke
Neuroprotective Agents
Streptozocin
alpha-Synuclein
Figure
Reference
-
1. Donnan GA, Fisher M, Macleod M, Davis SM. Stroke. Lancet. 2008; 371(9624):1612–1623.
Article2. Jiang YF, Liu ZQ, Cui W, Zhang WT, Gong JP, Wang XM, Zhang Y, Yang MJ. Antioxidant effect of salvianolic acid B on hippocampal CA1 neurons in mice with cerebral ischemia and reperfusion injury. Chin J Integr Med. 2015; 21(7):516–522.
Article3. Chen H, Yoshioka H, Kim GS, Jung JE, Okami N, Sakata H, Maier CM, Narasimhan P, Goeders CE, Chan PH. Oxidative stress in ischemic brain damage: mechanisms of cell death and potential molecular targets for neuroprotection. Antioxid Redox Signal. 2011; 14(8):1505–1517.
Article4. Kleikers PW, Wingler K, Hermans JJ, Diebold I, Altenhöfer S, Radermacher KA, Janssen B, Görlach A, Schmidt HH. NADPH oxidases as a source of oxidative stress and molecular target in ischemia/reperfusion injury. J Mol Med (Berl). 2012; 90(12):1391–1406.
Article5. Lee WC, Wong HY, Chai YY, Shi CW, Amino N, Kikuchi S, Huang SH. Lipid peroxidation dysregulation in ischemic stroke: plasma 4-HNE as a potential biomarker? Biochem Biophys Res Commun. 2012; 425(4):842–847.
Article6. Nigam S, Schewe T. Phospholipase A(2)s and lipid peroxidation. Biochim Biophys Acta. 2000; 1488(1-2):167–181.
Article7. Green DR, Reed JC. Mitochondria and apoptosis. Science. 1998; 281(5381):1309–1312.8. Kuribayashi Y, Yoshida K, Sakaue T, Okumura A. In vitro studies on the influence of L-ascorbic acid 2-[3,4-dihydro- 2,5,7,8-tetramethyl-2-(4,8,12-trimethyltridecyl)-2H-1-benzopyran-6yl-hy drogen phosphate] potassium salt on lipid peroxidation and phospholipase A2 activity. Arzneimittelforschung. 1992; 42(9):1072–1074.9. Block F, Kunkel M, Sontag KH. Posttreatment with EPC-K1, an inhibitor of lipid peroxidation and of phospholipase A2 activity, reduces functional deficits after global ischemia in rats. Brain Res Bull. 1995; 36(3):257–260.
Article10. Sidhu A, Wersinger C, Vernier P. Does alpha-synuclein modulate dopaminergic synaptic content and tone at the synapse? FASEB J. 2004; 18(6):637–647.11. Zhu M, Qin ZJ, Hu D, Munishkina LA, Fink AL. Alpha-synuclein can function as an antioxidant preventing oxidation of unsaturated lipid in vesicles. Biochemistry. 2006; 45(26):8135–8142.12. Volpicelli-Daley LA, Luk KC, Patel TP, Tanik SA, Riddle DM, Stieber A, Meaney DF, Trojanowski JQ, Lee VM. Exogenous α-synuclein fibrils induce Lewy body pathology leading to synaptic dysfunction and neuron death. Neuron. 2011; 72(1):57–71.
Article13. Luk KC, Kehm V, Carroll J, Zhang B, O'Brien P, Trojanowski JQ, Lee VM. Pathological α-synuclein transmission initiates Parkinson-like neurodegeneration in nontransgenic mice. Science. 2012; 338(6109):949–953.
Article14. Makoto H, Eliezer M. Alpha-synuclein in Lewy Body Disease and Alzheimer's Disease. Brain Pathol. 1999; 9:707–720.15. Newell KL, Boyer P, Gomez-Tortosa E, Hobbs W, Hedley-Whyte ET, Vonsattel JP, Hyman BT. Alpha-synuclein immunoreactivity is present in axonal swellings in neuroaxonal dystrophy and acute traumatic brain injury. J Neuropathol Exp Neurol. 1999; 58(12):1263–1268.16. Capes SE, Hunt D, Malmberg K, Pathak P, Gerstein HC. Stress hyperglycemia and prognosis of stroke in nondiabetic and diabetic patients: a systematic overview. Stroke. 2001; 32(10):2426–2432.17. Li ZG, Britton M, Sima AA, Dunbar JC. Diabetes enhances apoptosis induced by cerebral ischemia. Life Sci. 2004; 76(3):249–262.
Article18. Rizk NN, Rafols J, Dunbar JC. Cerebral ischemia induced apoptosis and necrosis in normal and diabetic rats. Brain Res. 2005; 1053(1-2):1–9.
Article19. Dietrich WD, Alonso O, Busto R. Moderate hyperglycemia worsens acute blood-brain barrier injury after forebrain ischemia in rats. Stroke. 1993; 24(1):111–116.
Article20. Bruno A, Liebeskind D, Hao Q, Raychev R;. Diabetes mellitus, acute hyperglycemia, and ischemic stroke. Curr Treat Options Neurol. 2010; 12(6):492–503.
Article21. Koh PO. Cerebral ischemic injury decreases α-synuclein expression in brain tissue and glutamate-exposed HT22 cells. Lab Anim Res. 2017; 33(3):244–250.
Article22. Tancrède G, Rousseau-Migneron S, Nadeau A. Long-term changes in the diabetic state induced by different doses of streptozotocin in rats. Br J Exp Pathol. 1983; 64(2):117–123.23. Ezquer M, Urzua CA, Montecino S, Leal K, Conget P, Ezquer F. Intravitreal administration of multipotent mesenchymal stromal cells triggers a cytoprotective microenvironment in the retina of diabetic mice. Stem Cell Res Ther. 2016; 7:42.
Article24. Longa EZ, Weinstein PR, Carlson S, Cummins R. Reversible middle cerebral artery occlusion without craniectomy in rats. Stroke. 1989; 20(1):84–91.
Article25. Shah FA, Park DJ, Koh PO. Identification of Proteins Differentially Expressed by Quercetin Treatment in a Middle Cerebral Artery Occlusion Model: A Proteomics Approach. Neurochem Res. 2018; 43:1608–1623.
Article26. Ye X, Chopp M, Cui X, Zacharek A, Cui Y, Yan T, Shehadah A, Roberts C, Liu X, Lu M, Chen J. Niaspan enhances vascular remodeling after stroke in type 1 diabetic rats. Exp Neurol. 2011; 232(2):299–308.
Article27. Ning R, Chopp M, Zacharek A, Yan T, Zhang C, Roberts C, Lu M, Chen J. Neamine induces neuroprotection after acute ischemic stroke in type one diabetic rats. Neuroscience. 2014; 257:76–85.
Article28. Alessandri JM, Guesnet P, Vancassel S, Astorg P, Denis I, Langelier B, Aïd S, Poumès-Ballihaut C, Champeil-Potokar G, Lavialle M. Polyunsaturated fatty acids in the central nervous system: evolution of concepts and nutritional implications throughout life. Reprod Nutr Dev. 2004; 44(6):509–538.
Article29. Sharon R, Bar-Joseph I, Frosch MP, Walsh DM, Hamilton JA, Selkoe DJ. The formation of highly soluble oligomers of alpha-synuclein is regulated by fatty acids and enhanced in Parkinson's disease. Neuron. 2003; 37(4):583–595.30. Qin Z, Hu D, Han S, Reaney SH, Di Monte DA, Fink AL. Effect of 4-hydroxy-2-nonenal modification on alpha-synuclein aggregation. J Biol Chem. 2007; 282(8):5862–5870.31. Kaplan B, Ratner V, Haas E. Alpha-synuclein: its biological function and role in neurodegenerative diseases. J Mol Neurosci. 2003; 20(2):83–92.32. Ma QL, Chan P, Yoshii M, Uéda K. Alpha-synuclein aggregation and neurodegenerative diseases. J Alzheimers Dis. 2003; 5(2):139–148.33. Lee M, Hyun D, Halliwell B, Jenner P. Effect of the overexpression of wild-type or mutant alpha-synuclein on cell susceptibility to insult. J Neurochem. 2001; 76(4):998–1009.34. Pompella A, Visvikis A, Paolicchi A, De Tata V, Casini AF. The changing faces of glutathione, a cellular protagonist. Biochem Pharmacol. 2003; 66(8):1499–1503.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Cerebral ischemic injury decreases α-synuclein expression in brain tissue and glutamate-exposed HT22 cells
- Hyperglycemia exacerbates downregulation of dynamin-like protein 1 in ischemic cerebral injury
- Hyperglycemia decreases preoxiredoxin-2 expression in a middle cerebral artery occlusion model
- The Time Evolution of Cerebral Apoptosis in the Permanent Middle Cerebral Artery Occlusion Model in Rats
- Intracranial Cerebrovascular Revascularization(Extracranial-Intracranial Arterial Bypass, EIAB)