Allergy Asthma Immunol Res.
2019 Nov;11(6):856-870. 10.4168/aair.2019.11.6.856.
Inhaled Corticosteroids and Placebo Treatment Effects in Adult Patients With Cough: A Systematic Review and Meta-analysis
- Affiliations
-
- 1Department of Internal Medicine, Pusan National University Yangsan Hospital, Yangsan, Korea.
- 2Department of Allergy and Clinical Immunology, Airway Sensation and Cough Research Laboratory, Asan Medical Center, University of Ulsan College of Medicine, Seoul, Korea. swj0126@amc.seoul.kr
- 3Department of Preventive Medicine, College of Medicine, Korea University, Seoul, Korea.
- 4Division of Allergy, Department of Medicine, Samsung Medical Center, Sungkyunkwan University School of Medicine, Seoul, Korea.
- 5Division of Allergy and Clinical Immunology, Department of Internal Medicine, Seoul National University College of Medicine, Seoul, Korea.
- 6Optimum Patient Care, Cambridge, United Kingdom; Centre of Academic Primary Care, Division of Applied Health Sciences, University of Aberdeen, Aberdeen, United Kingdom.
- 7Centre for Cardiovascular and Metabolic Research, Hull York Medical School, Castle Hill Hospital, University of Hull, Cottingham, East Yorkshire, United Kingdom.
- KMID: 2459189
- DOI: http://doi.org/10.4168/aair.2019.11.6.856
Abstract
- PURPOSE
Inhaled corticosteroids (ICSs) are often considered an empirical therapy in the management of patients with cough. However, ICS responsiveness is difficult to interpret in daily clinical practice, as the improvements may include placebo effects or self-remission. We aimed to evaluate ICS and placebo treatment effects in adult patients with cough.
METHODS
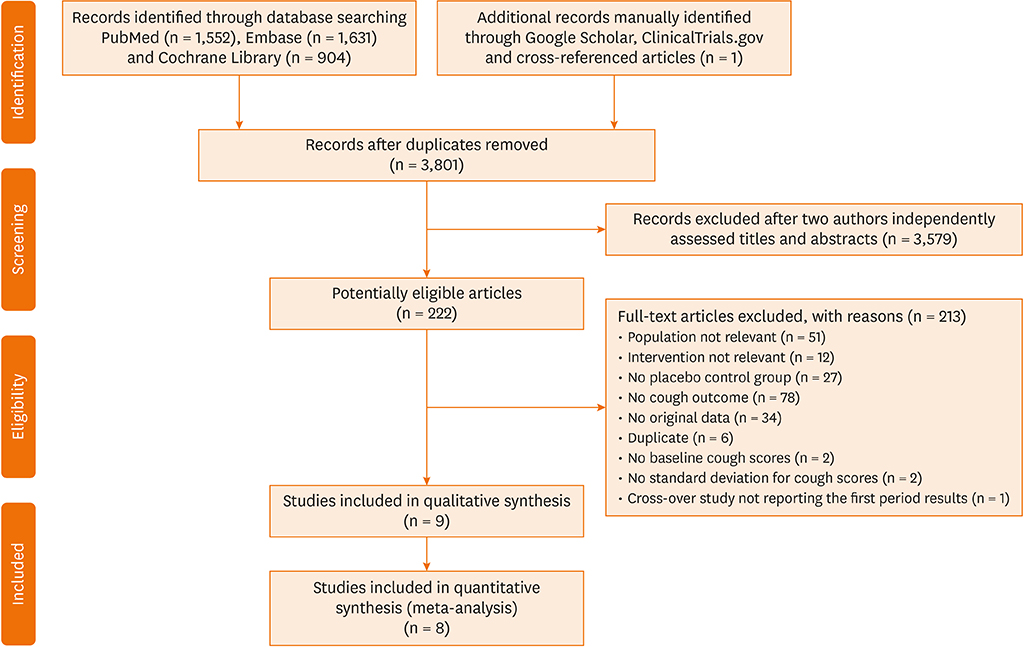
Electronic databases were searched for studies published until June 2018, without language restriction. Randomized controlled trials reporting the effects of ICSs compared with placebo in adult patients with cough were included. Random effects meta-analyses were conducted to estimate the treatment effects. Therapeutic gain was calculated by subtracting the percentage change from baseline in the cough score in the ICS treatment group from that in the placebo treatment group.
RESULTS
A total of 9 studies were identified and 8 studies measuring cough severity outcomes were included for meta-analyses. Therapeutic gain from ICSs ranged from −5.0% to +94.6% across the studies included; however, it did not exceed +22%, except for an outlier reporting very high therapeutic gains (+45.6% to +94.6%, depending on outcomes). Overall ICS treatment effects in cough severity outcomes were small-to-moderate (standardized mean difference [SMD], −0.38; 95% confidence interval [CI], −0.54, −0.23), which were comparable between subacute and chronic coughs. However, pooled placebo treatment effects were very large in subacute cough (SMD, −2.58; 95% CI, −3.03, −2.1), and modest but significant in chronic cough (SMD, −0.46; 95% CI, −0.72, −0.21).
CONCLUSIONS
Overall therapeutic gain from ICSs is small-to-moderate. However, placebo treatment effects of ICS are large in subacute cough, and modest but still significant in chronic cough. These findings indicate the need for careful interpretation of ICS responsiveness in the management of cough patients in the clinic, and also for rigorous patient selection to identify ICS-responders.
Keyword
MeSH Terms
Figure
Cited by 1 articles
-
New ERS cough guidelines: A clinical framework for refining the patient management strategy
Woo-Jung Song, Eva Millqvist, Alyn H. Morice
Asia Pac Allergy. 2019;9(4):. doi: 10.5415/apallergy.2019.9.e36.
Reference
-
1. Song WJ, Morice AH. Cough hypersensitivity syndrome: a few more steps forward. Allergy Asthma Immunol Res. 2017; 9:394–402.
Article2. Morice AH. Epidemiology of cough. Pulm Pharmacol Ther. 2002; 15:253–259.
Article3. Cho SH, Lin HC, Ghoshal AG, Bin Abdul Muttalif AR, Thanaviratananich S, Bagga S, et al. Respiratory disease in the Asia-Pacific region: cough as a key symptom. Allergy Asthma Proc. 2016; 37:131–140.
Article4. Song WJ, Chang YS, Faruqi S, Kim JY, Kang MG, Kim S, et al. The global epidemiology of chronic cough in adults: a systematic review and meta-analysis. Eur Respir J. 2015; 45:1479–1481.
Article5. French CL, Irwin RS, Curley FJ, Krikorian CJ. Impact of chronic cough on quality of life. Arch Intern Med. 1998; 158:1657–1661.
Article6. Morice AH, Fontana GA, Sovijarvi AR, Pistolesi M, Chung KF, Widdicombe J, et al. The diagnosis and management of chronic cough. Eur Respir J. 2004; 24:481–492.
Article7. Vertigan AE, Haines J, Slovarp L. An update on speech pathology management of chronic refractory cough. J Allergy Clin Immunol Pract. 2019; 7:1756–1761.
Article8. Ryan NM, Vertigan AE, Birring SS. An update and systematic review on drug therapies for the treatment of refractory chronic cough. Expert Opin Pharmacother. 2018; 19:687–711.
Article9. Irwin RS, French CL, Chang AB, Altman KW. CHEST Expert Cough Panel*. Classification of cough as a symptom in adults and management algorithms: CHEST guideline and expert panel report. Chest. 2018; 153:196–209.10. Kwon NH, Oh MJ, Min TH, Lee BJ, Choi DC. Causes and clinical features of subacute cough. Chest. 2006; 129:1142–1147.
Article11. Brightling CE, Ward R, Goh KL, Wardlaw AJ, Pavord ID. Eosinophilic bronchitis is an important cause of chronic cough. Am J Respir Crit Care Med. 1999; 160:406–410.
Article12. Crapo RO, Casaburi R, Coates AL, Enright PL, Hankinson JL, Irvin CG, et al. Guidelines for methacholine and exercise challenge testing-1999. This official statement of the American Thoracic Society was adopted by the ATS board of directors, July 1999. Am J Respir Crit Care Med. 2000; 161:309–329.13. Djukanović R, Sterk PJ, Fahy JV, Hargreave FE. Standardised methodology of sputum induction and processing. Eur Respir J Suppl. 2002; 37:1s–2s.14. Song DJ, Song WJ, Kwon JW, Kim GW, Kim MA, Kim MY, et al. KAAACI evidence-based clinical practice guidelines for chronic cough in adults and children in Korea. Allergy Asthma Immunol Res. 2018; 10:591–613.
Article15. Johnstone KJ, Chang AB, Fong KM, Bowman RV, Yang IA. Inhaled corticosteroids for subacute and chronic cough in adults. Cochrane Database Syst Rev. 2013; CD009305.
Article16. Irwin RS, Baumann MH, Bolser DC, Boulet LP, Braman SS, Brightling CE, et al. Diagnosis and management of cough executive summary: ACCP evidence-based clinical practice guidelines. Chest. 2006; 129:1S–23S.17. Moher D, Liberati A, Tetzlaff J, Altman DG. PRISMA Group. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. BMJ. 2009; 339:b2535.
Article18. Glanville JM, Lefebvre C, Miles JN, Camosso-Stefinovic J. How to identify randomized controlled trials in MEDLINE: ten years on. J Med Libr Assoc. 2006; 94:130–136.19. Higgins JP, Altman DG, Gøtzsche PC, Jüni P, Moher D, Oxman AD, et al. The Cochrane Collaboration's tool for assessing risk of bias in randomised trials. BMJ. 2011; 343:d5928.
Article20. Higgins JPT, Green S. Cochrane handbook for systematic reviews of interventions. Hoboken, NJ: Wiley;2008.21. Cohen J. Statistical power analysis for the behavioral sciences. 2nd ed. Abingdon: Routledge;2013.22. Boulet LP, Milot J, Boutet M, St Georges F, Laviolette M. Airway inflammation in nonasthmatic subjects with chronic cough. Am J Respir Crit Care Med. 1994; 149:482–489.
Article23. Pizzichini MM, Pizzichini E, Parameswaran K, Clelland L, Efthimiadis A, Dolovich J, et al. Nonasthmatic chronic cough: no effect of treatment with an inhaled corticosteroid in patients without sputum eosinophilia. Can Respir J. 1999; 6:323–330.24. Rytilä P, Ghaly L, Varghese S, Chung W, Selroos O, Haahtela T, et al. Treatment with inhaled steroids in patients with symptoms suggestive of asthma but with normal lung function. Eur Respir J. 2008; 32:989–996.25. Pornsuriyasak P, Charoenpan P, Vongvivat K, Thakkinstian A. Inhaled corticosteroid for persistent cough following upper respiratory tract infection. Respirology. 2005; 10:520–524.
Article26. Ponsioen BP, Hop WC, Vermue NA, Dekhuijzen PN, Bohnen AM. Efficacy of fluticasone on cough: a randomised controlled trial. Eur Respir J. 2005; 25:147–152.
Article27. Price DB, Buhl R, Chan A, Freeman D, Gardener E, Godley C, et al. Fractional exhaled nitric oxide as a predictor of response to inhaled corticosteroids in patients with non-specific respiratory symptoms and insignificant bronchodilator reversibility: a randomised controlled trial. Lancet Respir Med. 2018; 6:29–39.
Article28. Ribeiro M, Pereira CA, Nery LE, Beppu OS, Silva CO. High-dose inhaled beclomethasone treatment in patients with chronic cough: a randomized placebo-controlled study. Ann Allergy Asthma Immunol. 2007; 99:61–68.
Article29. Gillissen A, Richter A, Oster H. Clinical efficacy of short-term treatment with extra-fine HFA beclomethasone dipropionate in patients with post-infectious persistent cough. J Physiol Pharmacol. 2007; 58 Suppl 5:223–232.30. Engel T, Heinig JH, Madsen O, Hansen M, Weeke ER. A trial of inhaled budesonide on airway responsiveness in smokers with chronic bronchitis. Eur Respir J. 1989; 2:935–939.31. Chaudhuri R, McMahon AD, Thomson LJ, MacLeod KJ, McSharry CP, Livingston E, et al. Effect of inhaled corticosteroids on symptom severity and sputum mediator levels in chronic persistent cough. J Allergy Clin Immunol. 2004; 113:1063–1070.
Article32. Wesseling GJ, Quaedvlieg M, Wouters EF. Inhaled budesonide in chronic bronchitis. Effects on respiratory impedance. Eur Respir J. 1991; 4:1101–1105.33. Evald T, Munch EP, Kok-Jensen A. Chronic non-asthmatic cough is not affected by inhaled beclomethasone dipropionate. A controlled double blind clinical trial. Allergy. 1989; 44:510–514.34. van Laarhoven AI, van der Sman-Mauriks IM, Donders AR, Pronk MC, van de Kerkhof PC, Evers AW. Placebo effects on itch: a meta-analysis of clinical trials of patients with dermatological conditions. J Invest Dermatol. 2015; 135:1234–1243.
Article35. Häuser W, Sarzi-Puttini P, Tölle TR, Wolfe F. Placebo and nocebo responses in randomised controlled trials of drugs applying for approval for fibromyalgia syndrome treatment: systematic review and meta-analysis. Clin Exp Rheumatol. 2012; 30:78–87.36. Miranda Varella Pereira G, Soriano Marcolino M, Silveira Nogueira Reis Z, Vale de Castro Monteiro M. A systematic review of drug treatment of vulvodynia: evidence of a strong placebo effect. BJOG. 2018; 125:1216–1224.37. Vase L, Vollert J, Finnerup NB, Miao X, Atkinson G, Marshall S, et al. Predictors of the placebo analgesia response in randomized controlled trials of chronic pain: a meta-analysis of the individual data from nine industrially sponsored trials. Pain. 2015; 156:1795–1802.38. Elsenbruch S, Enck P. Placebo effects and their determinants in gastrointestinal disorders. Nat Rev Gastroenterol Hepatol. 2015; 12:472–485.
Article39. Eccles R. Importance of placebo effect in cough clinical trials. Lung. 2010; 188 Suppl 1:S53–S61.
Article40. Gibson PG, Dolovich J, Denburg J, Ramsdale EH, Hargreave FE. Chronic cough: eosinophilic bronchitis without asthma. Lancet. 1989; 1:1346–1348.
Article41. Agache I, Rogozea L. Asthma biomarkers: do they bring precision medicine closer to the clinic? Allergy Asthma Immunol Res. 2017; 9:466–476.
Article42. Song WJ, Kim HJ, Shim JS, Won HK, Kang SY, Sohn KH, et al. Diagnostic accuracy of fractional exhaled nitric oxide measurement in predicting cough-variant asthma and eosinophilic bronchitis in adults with chronic cough: a systematic review and meta-analysis. J Allergy Clin Immunol. 2017; 140:701–709.43. Smith AD, Cowan JO, Brassett KP, Filsell S, McLachlan C, Monti-Sheehan G, et al. Exhaled nitric oxide: a predictor of steroid response. Am J Respir Crit Care Med. 2005; 172:453–459.44. Yi F, Chen R, Luo W, Xu D, Han L, Liu B, et al. Validity of fractional exhaled nitric oxide in diagnosis of corticosteroid-responsive cough. Chest. 2016; 149:1042–1051.
Article45. Inoue Y, Sato S, Manabe T, Makita E, Chiyotanda M, Takahashi K, et al. Measurement of exhaled nitric oxide in children: a comparison between NObreath® and NIOX VERO® analyzers. Allergy Asthma Immunol Res. 2018; 10:478–489.46. Birring SS, Spinou A. How best to measure cough clinically. Curr Opin Pharmacol. 2015; 22:37–40.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- An Introduction of the Systematic Review and Meta-Analysis
- Effect of premedication on postoperative pain after root canal therapy in patients with irreversible pulpitis: a systematic review and meta-analysis
- Concepts and emerging issues of network meta-analysis
- Critical Appraisal of Systematic Review/Meta-analysis
- Effectiveness of Intratympanic Dexamethasone Injection for Tinnitus Treatment: A Systematic Review and Meta-Analysis