Yonsei Med J.
2019 Oct;60(10):905-913. 10.3349/ymj.2019.60.10.905.
The Long Non-Coding RNA CASC2 Suppresses Cell Viability, Migration, and Invasion in Hepatocellular Carcinoma Cells by Directly Downregulating miR-183
- Affiliations
-
- 1Department II of General Surgery, The People's Hospital of Hanchuan, Hanchuan, Hubei, China. gaoshanliushuiyzj@126.com
- 2Department of Gynaecology and Obstetrics, The People's Hospital of Hanchuan, Hanchuan, Hubei, China.
- KMID: 2459143
- DOI: http://doi.org/10.3349/ymj.2019.60.10.905
Abstract
- PURPOSE
Hepatocellular carcinoma (HCC) is the most common malignant tumor of liver cells. Researchers have reported that cancer susceptibility candidate 2 (CASC2), a long non-coding RNA, is down-regulated in various cancers, including HCC. Our study aimed to investigate the molecular mechanism(s) of CASC2 in HCC.
MATERIALS AND METHODS
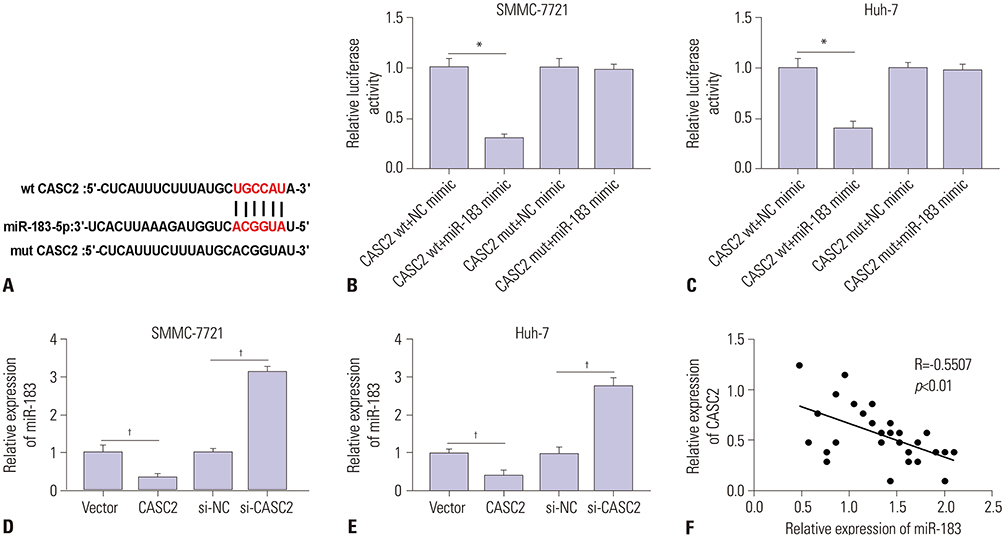
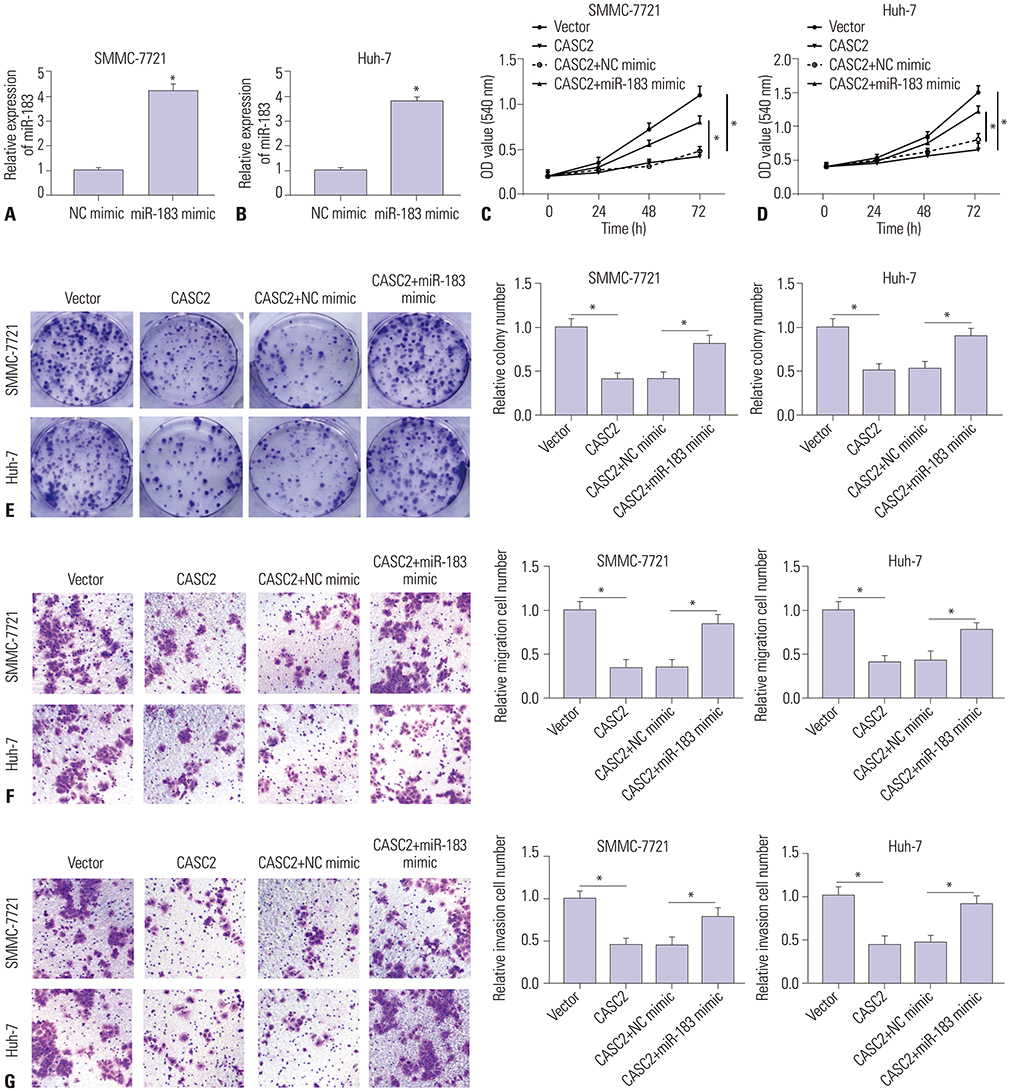
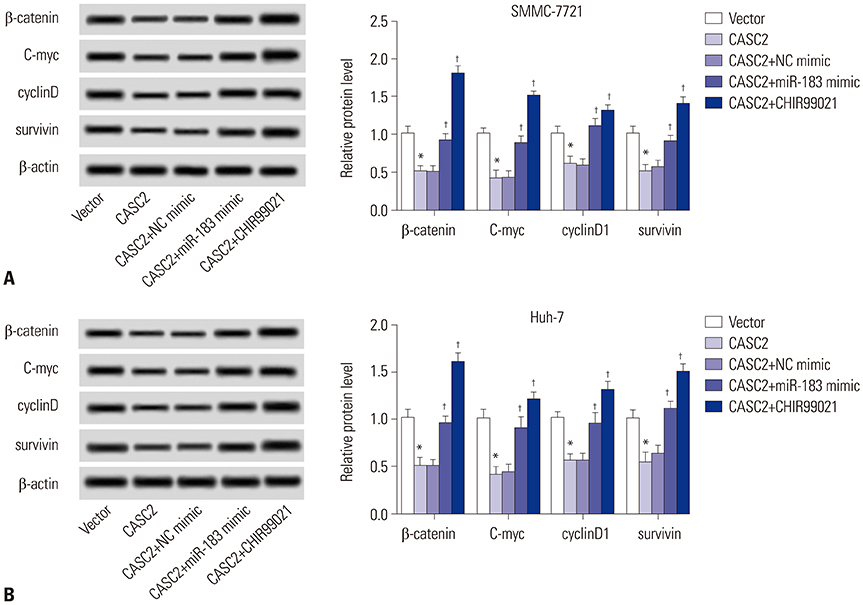
Real-time quantitative PCR (RT-qPCR) was used to analyze the expression of CASC2 and miR-183 in HCC tissues and cells. The viability of HCC SMMC-7721 and Huh-7 cells was detected through MTT assay. Colony formation assay was performed to assess the colony formation ability of HCC cells. The migration and invasion abilities of HCC cells were evaluated by Transwell assay. Western blot was conducted to examine levels of key Wnt/β-catenin signaling pathway factors, C-myc, cyclinD, survivin, and β-catenin. The interaction between CASC2 and miR-183 was affirmed by bioinformatics analysis and luciferase reporter assay.
RESULTS
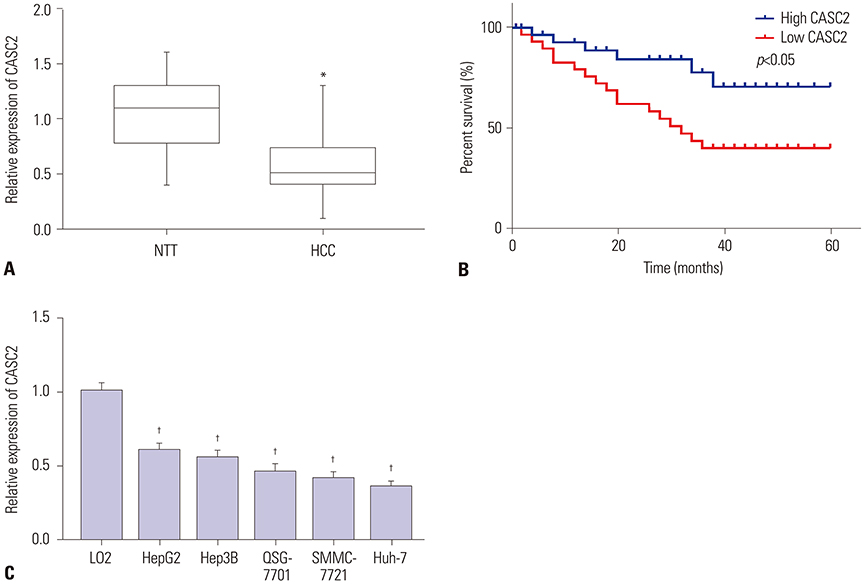
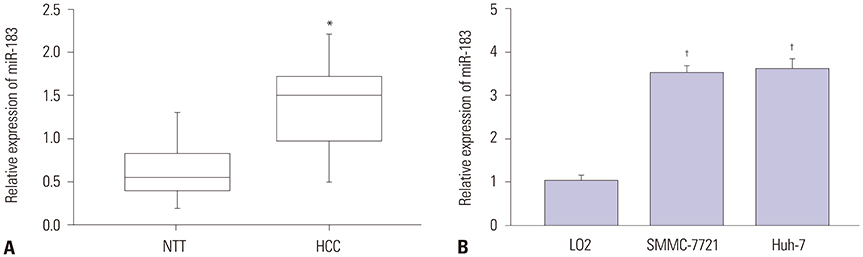
CASC2 was down-regulated in HCC tissues and cell lines, while miR-183 was up-regulated. The expression of miR-183 was negatively correlated with CASC2 expression in HCC tissues. Overexpression of CASC2 inhibited cell viability, colony formation, migration, and invasion in HCC cells, as well as Wnt/β-catenin signaling pathway activity. miR-183 was a downstream target of CASC2 and negatively regulated by CASC2. Introduction of miR-183 rescued CASC2-induced suppressive effects on HCC cell viability, colony formation, migration, and invasion and Wnt/β-catenin signaling.
CONCLUSION
CASC2 inhibited cell viability and the colony formation, migration, and invasion abilities of HCC cells by directly downregulating miR-183 through inactivation of the Wnt/β-catenin signaling pathway.
MeSH Terms
Figure
Reference
-
1. Coskun M. Hepatocellular carcinoma in the cirrhotic liver: evaluation using computed tomography and magnetic resonance imaging. Exp Clin Transplant. 2017; 15(Suppl 2):36–44.2. Khemlina G, Ikeda S, Kurzrock R. The biology of Hepatocellular carcinoma: implications for genomic and immune therapies. Mol Cancer. 2017; 16:149.
Article3. Mercer TR, Dinger ME, Mattick JS. Long non-coding RNAs: insights into functions. Nat Rev Genet. 2009; 10:155–159.
Article4. Mehra M, Chauhan R. Long noncoding RNAs as a key player in hepatocellular carcinoma. Biomark Cancer. 2017; 9:1179299X17737301.
Article5. Li C, Chen J, Zhang K, Feng B, Wang R, Chen L. Progress and prospects of long noncoding RNAs (lncRNAs) in hepatocellular carcinoma. Cell Physiol Biochem. 2015; 36:423–434.
Article6. Baldinu P, Cossu A, Manca A, Satta MP, Sini MC, Rozzo C, et al. Identification of a novel candidate gene, CASC2, in a region of common allelic loss at chromosome 10q26 in human endometrial cancer. Hum Mutat. 2004; 23:318–326.
Article7. Baldinu P, Cossu A, Manca A, Satta MP, Sini MC, Palomba G, et al. CASC2a gene is down-regulated in endometrial cancer. Anticancer Res. 2007; 27(1A):235–243.8. Cai J, Zuo X, Chen Z, Zhao W, Zhu Y, Zhang Z, et al. Prognostic value and clinical significance of long noncoding RNA CASC2 in human malignancies: a meta-analysis. Cancer Manag Res. 2018; 10:1403–1412.
Article9. Yu X, Zheng H, Tse G, Zhang L, Wu WKK. CASC2: an emerging tumour-suppressing long noncoding RNA in human cancers and melanoma. Cell Prolif. 2018; 51:e12506.
Article10. Ba Z, Gu L, Hao S, Wang X, Cheng Z, Nie G. Downregulation of lncRNA CASC2 facilitates osteosarcoma growth and invasion through miR-181a. Cell Prolif. 2018; 51:DOI: 10.1111/cpr.12409.
Article11. Pei Z, Du X, Song Y, Fan L, Li F, Gao Y, et al. Down-regulation of lncRNA CASC2 promotes cell proliferation and metastasis of bladder cancer by activation of the Wnt/β-catenin signaling pathway. Oncotarget. 2017; 8:18145–18153.
Article12. Wang R, Li Y, Zhu G, Tian B, Zeng W, Yang Y, et al. Long noncoding RNA CASC2 predicts the prognosis of glioma patients and functions as a suppressor for gliomas by suppressing Wnt/β-catenin signaling pathway. Neuropsychiatr Dis Treat. 2017; 13:1805–1813.13. Gan Y, Han N, He X, Yu J, Zhang M, Zhou Y, et al. Long non-coding RNA CASC2 regulates cell biological behaviour through the MAPK signalling pathway in hepatocellular carcinoma. Tumour Biol. 2017; 39:1010428317706229.
Article14. Zhao L, Zhang Y, Zhang Y. Long noncoding RNA CASC2 regulates hepatocellular carcinoma cell oncogenesis through miR-362-5p/Nf-κB axis. J Cell Physiol. 2018; 233:6661–6670.
Article15. Hayes CN, Chayama K. MicroRNAs as biomarkers for liver disease and hepatocellular carcinoma. Int J Mol Sci. 2016; 17:280.
Article16. Sohn W, Kim J, Kang SH, Yang SR, Cho JY, Cho HC, et al. Serum exosomal microRNAs as novel biomarkers for hepatocellular carcinoma. Exp Mol Med. 2015; 47:e184.
Article17. Liu LN, Li DD, Xu HX, Zheng SG, Zhang XP. Role of microRNAs in hepatocellular carcinoma. Front Biosci (Landmark Ed). 2015; 20:1056–1067.
Article18. Anwar SL, Krech T, Hasemeier B, Schipper E, Schweitzer N, Vogel A, et al. hsa-mir-183 is frequently methylated and related to poor survival in human hepatocellular carcinoma. World J Gastroenterol. 2017; 23:1568–1575.
Article19. Clevers H, Nusse R. Wnt/β-catenin signaling and disease. Cell. 2012; 149:1192–1205.
Article20. Pai SG, Carneiro BA, Mota JM, Costa R, Leite CA, Barroso-Sousa R, et al. Wnt/beta-catenin pathway: modulating anticancer immune response. J Hematol Oncol. 2017; 10:101.
Article21. Leung WK, He M, Chan AW, Law PT, Wong N. Wnt/beta-Catenin activates MiR-183/96/182 expression in hepatocellular carcinoma that promotes cell invasion. Cancer Lett. 2015; 362:97–105.
Article22. Song Y, Wang F, Huang Q, Cao Y, Zhao Y, Yang C. MicroRNAs contribute to hepatocellular carcinoma. Mini Rev Med Chem. 2015; 15:459–466.
Article23. Ozer Etik D, Suna N, Boyacioglu AS. Management of hepatocellular carcinoma: prevention, surveillance, diagnosis, and staging. Exp Clin Transplant. 2017; 15(Suppl 2):31–35.24. Liu WT, Lu X, Tang GH, Ren JJ, Liao WJ, Ge PL, et al. LncRNAs expression signatures of hepatocellular carcinoma revealed by microarray. World J Gastroenterol. 2014; 20:6314–6321.
Article25. Zhu ZM, Liu FT, Chen X. Low expression of LncRNA cancer susceptibility candidate 2 and its clinical significance in cancer tissues. Cell Physiol Biochem. 2018; 46:1643–1649.
Article26. Fan JC, Zeng F, Le YG, Xin L. LncRNA CASC2 inhibited the viability and induced the apoptosis of hepatocellular carcinoma cells through regulating miR-24-3p. J Cell Biochem. 2018; 119:6391–6397.
Article27. Li J, Fu H, Xu C, Tie Y, Xing R, Zhu J, et al. miR-183 inhibits TGF-beta1-induced apoptosis by downregulation of PDCD4 expression in human hepatocellular carcinoma cells. BMC Cancer. 2010; 10:354.
Article28. Dambal S, Shah M, Mihelich B, Nonn L. The microRNA-183 cluster: the family that plays together stays together. Nucleic Acids Res. 2015; 43:7173–7188.
Article29. Ma Y, Liang AJ, Fan YP, Huang YR, Zhao XM, Sun Y, et al. Dysregulation and functional roles of miR-183-96-182 cluster in cancer cell proliferation, invasion and metastasis. Oncotarget. 2016; 7:42805–42825.
Article30. Yang CL, Zheng XL, Ye K, Ge H, Sun YN, Lu YF, et al. MicroRNA-183 acts as a tumor suppressor in human non-small cell lung cancer by down-regulating MTA1. Cell Physiol Biochem. 2018; 46:93–106.
Article31. Wang J, Wang X, Li Z, Liu H, Teng Y. MicroRNA-183 suppresses retinoblastoma cell growth, invasion and migration by targeting LRP6. FEBS J. 2014; 281:1355–1365.
Article32. Xu L, Li Y, Yan D, He J, Liu D. MicroRNA-183 inhibits gastric cancer proliferation and invasion via directly targeting Bmi-1. Oncol Lett. 2014; 8:2345–2351.
Article33. Wang X, Zuo D, Yuan Y, Yang X, Hong Z, Zhang R. MicroRNA-183 promotes cell proliferation via regulating programmed cell death 6 in pediatric acute myeloid leukemia. J Cancer Res Clin Oncol. 2017; 143:169–180.
Article34. Xiong H, Chen R, Liu S, Lin Q, Chen H, Jiang Q. MicroRNA-183 induces epithelial-mesenchymal transition and promotes endometrial cancer cell migration and invasion in by targeting CPEB1. J Cell Biochem. 2018; 119:8123–8137.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Long Non-Coding RNA NORAD Inhibits Breast Cancer Cell Proliferation and Metastasis by Regulating miR-155-5p/ SOCS1 Axis
- Long Intergenic Non-Protein Coding RNA 665 Regulates Viability, Apoptosis, and Autophagy via the MiR-186-5p/MAP4K3 Axis in Hepatocellular Carcinoma
- AC092127.1-miR-451a-AE binding protein 2 Signaling Facilitates Malignant Properties of Breast Cancer
- Long Noncoding RNA MALAT1 Regulates Hepatocellular Carcinoma Growth Under Hypoxia via Sponging MicroRNA-200a
- Long Noncoding RNA PVT1 Promotes Stemness and Temozolomide Resistance through miR-365/ELF4/SOX2 Axis in Glioma