Transl Clin Pharmacol.
2019 Sep;27(3):107-114. 10.12793/tcp.2019.27.3.107.
Development of a physiologically-based pharmacokinetic model for cyclosporine in Asian children with renal impairment
- Affiliations
-
- 1Department of Clinical Pharmacology and Therapeutics, Seoul National University College of Medicine and Hospital, Seoul 03080, Republic of Korea.
- 2Department of Clinical Pharmacology and Therapeutics, Seoul National University College of Medicine and Bundang Hospital, Seongnam 13620, Republic of Korea. jychung@snubh.org
- KMID: 2458950
- DOI: http://doi.org/10.12793/tcp.2019.27.3.107
Abstract
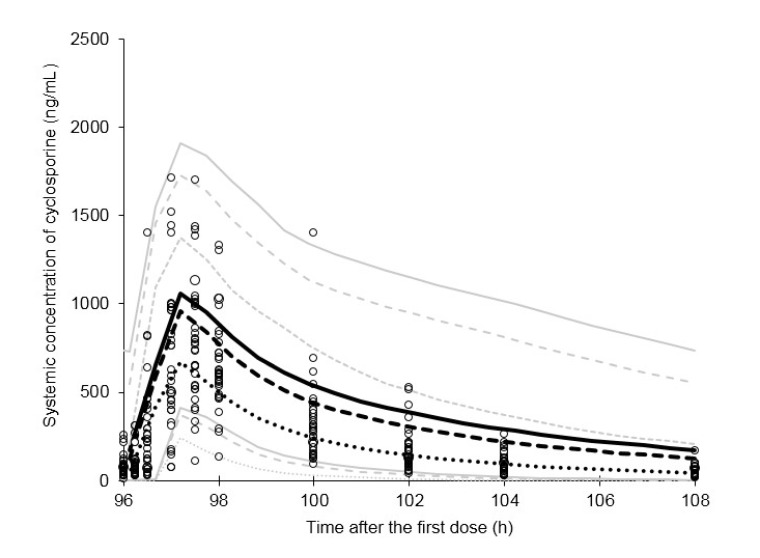
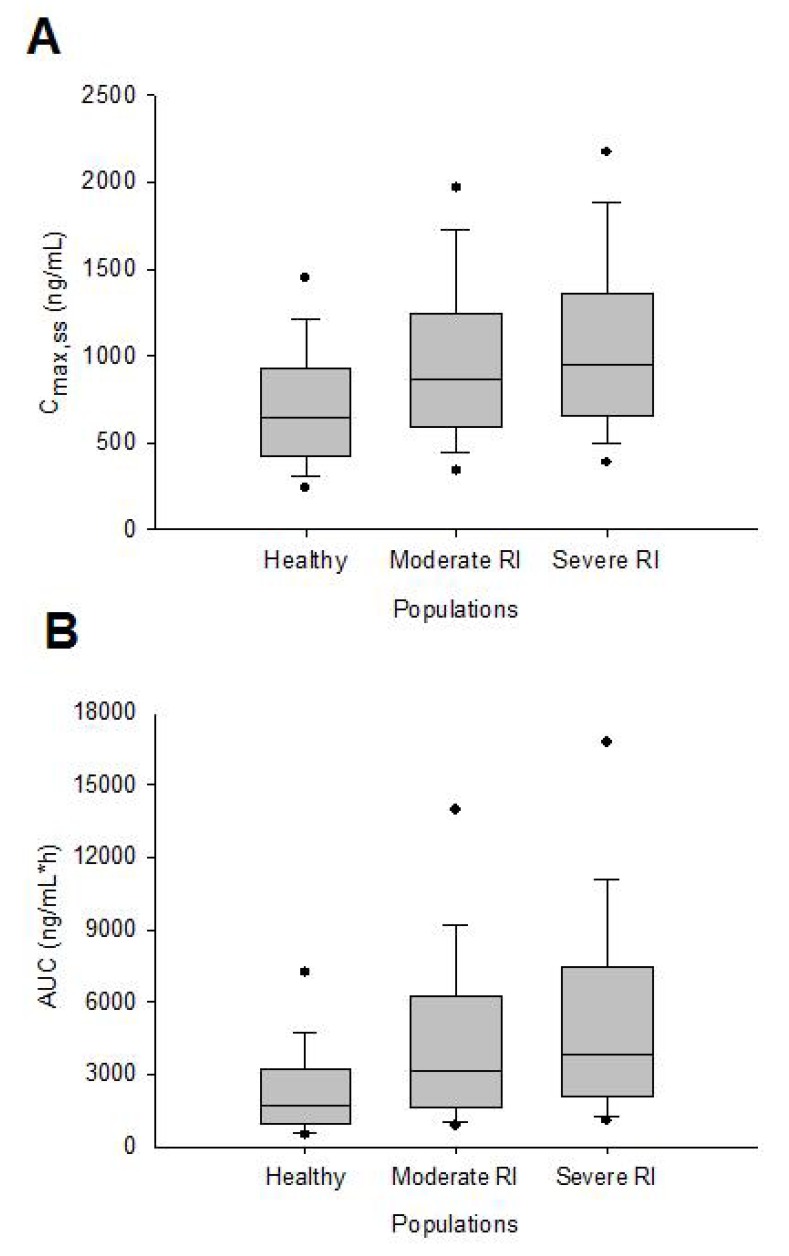
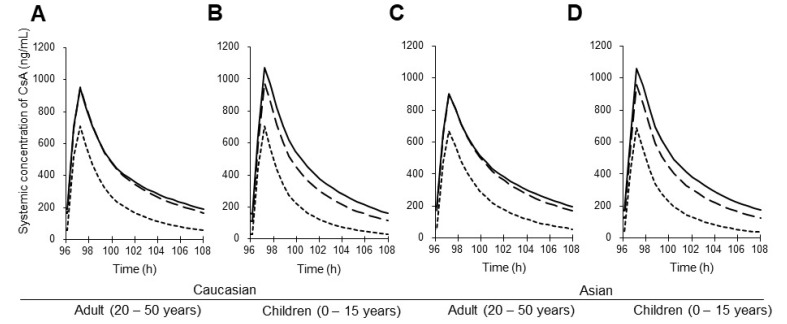
- This study aimed to assess the pharmacokinetics of cyclosporine A (CsA) in Asian children with renal impairment (RI) by developing a physiologically-based pharmacokinetic (PBPK) model with Simcyp Simulator. The PBPK model of Asian children with RI was developed by modifying the physiological parameters of the built-in population libraries in Simcyp Simulator. The ratio of healthy and RI populations was obtained for each parameter showing a difference between the populations. Each ratio was multiplied by the corresponding parameter in healthy Asian children. The model verification was performed with published data of Korean children with kidney disease given multiple CsA administrations. Simulations were performed with different combinations of ethnicity, age, and renal function to identify the net impact of each factor. The simulated results suggested that the effect of RI was higher in children than adults for both Caucasian and Asian. In conclusion, the constructed model adequately characterized CsA pharmacokinetics in Korean children with RI. Simulations with populations categorized by ethnicity, age, and renal function enabled to assess the net impact of each factor on specific populations.
Keyword
MeSH Terms
Figure
Reference
-
1. Henriques Ldos S, Matos Fde M, Vaisbich MH. Pharmacokinetics of cyclosporine - a microemulsion in children with idiopathic nephrotic syndrome. Clinics (Sao Paulo). 2012; 67:1197–1202. DOI: 10.6061/clinics/2012(10)12. PMID: 23070347.2. Hricik DE, O'Toole MA, Schulak JA, Herson J. Steroid-free immunosuppression in cyclosporine-treated renal transplant recipients: a meta-analysis. J Am Soc Nephrol. 1993; 4:1300–1305. DOI: 10.1034/j.1600-6143.2002.020105.x. PMID: 8130356.
Article3. Kidney Disease: Improving Global Outcomes (KDIGO) Glomerulonephritis Work Group. KDIGO Clinical Practice Guideline for Glomerulonephritis. Kidney Inter. 2012; 2:S139–S274. DOI: 10.1038/kisup.2012.10.4. Clardy CW, Schroeder TJ, Myre SA, Wadhwa NK, Pesce AJ, First MR, et al. Clinical variability of cyclosporine pharmacokinetics in adult and pediatric patients after renal, cardiac, hepatic, and bone-marrow transplants. Clin Chem. 1988; 34:2012–2015. PMID: 3048779.
Article5. del Mar Fernández De Gatta M, Santos-Buelga D, Domínguez-Gil A, García MJ. Immunosuppressive therapy for paediatric transplant patients: pharmacokinetic considerations. Clin Pharmacokinet. 2002; 41:115–135. PMID: 11888332.6. Min DI, Ellingrod VL, Marsh S, McLeod H. CYP3A5 polymorphism and the ethnic differences in cyclosporine pharmacokinetics in healthy subjects. Ther Drug Monit. 2004; 26:524–528. PMID: 15385835.7. Lindholm A, Welsh M, Alton C, Kahan BD. Demographic factors influencing cyclosporine pharmacokinetic parameters in patients with uremia: racial differences in bioavailability. Clin Pharmacol Ther. 1992; 52:359–371. PMID: 1330397.
Article8. Kimchi-Sarfaty C, Marple AH, Shinar S, Kimchi AM, Scavo D, Roma MI, et al. Ethnicity-related polymorphisms and haplotypes in the human ABCB1 gene. Pharmacogenomics. 2007; 8:29–39. DOI: 10.2217/14622416.8.1.29. PMID: 17187507.
Article9. Christians U, Strom T, Zhang YL, Steudel W, Schmitz V, Trump S, et al. Active drug transport of immunosuppressants: new insights for pharmacokinetics and pharmacodynamics. Ther Drug Monit. 2006; 28:39–44. PMID: 16418692.10. Lalande L, Charpiat B, Leboucher G, Tod M. Consequences of renal failure on non-renal clearance of drugs. Clin Pharmacokinet. 2014; 53:521–532. DOI: 10.1007/s40262-014-0146-1. PMID: 24861189.
Article11. Nolin TD. Altered nonrenal drug clearance in ESRD. Curr Opin Nephrol Hypertens. 2008; 17:555–559. DOI: 10.1097/MNH.0b013e3283136732. PMID: 18941346.
Article12. Yee GC, Lennon TP, Gmur DJ, Kennedy MS, Deeg HJ. Age-dependent cyclosporine: pharmacokinetics in marrow transplant recipients. Clin Pharmacol Ther. 1986; 40:438–443. PMID: 3530588.
Article13. Burckart G, Starzl T, Williams L, Sanghvi A, Gartner C, Venkataramanan R, et al. Cyclosporine monitoring and pharmacokinetics in pediatrie liver transplant patients. Transplant Proc. 1985; 17:1172–1175. PMID: 20640180.14. Cooney GF, Habucky K, Hoppu K. Cyclosporin pharmacokinetics in paediatric transplant recipients. Clin Pharmacokinet. 1997; 32:481–495. DOI: 10.2165/00003088-199732060-. PMID: 9195117.
Article15. Yee GC, Lennon TP, Gmur DG, Carlin J, Schaffer RL, Kennedy MS, et al. Clinical pharmacology of cyclosporine in patients undergoing bone marrow transplantation. Transplant Proc. 1986; 18(6 Suppl 5):153–159. PMID: 3538566.16. Sandimmune® (cyclosporine) capsules [package insert]. East Hanover (NJ): Novartis Pharmaceuticals Corporation;2015. cited 2017 Sep 05. Available from: https://www.pharma.us.novartis.com/sites/www.pharma.us.novartis.com/files/sandimmune.pdf.17. Jin M, Seto W, Taylor T, Saunders EF, Doyle J, Dupuis LL. Determination of initial i.v. CYA dosage to achieve target AUC values in pediatric hematopoietic stem cell transplant patients. Bone Marrow Transplant. 2008; 42:455–459. DOI: 10.1038/bmt.2008.189. PMID: 18622423.
Article18. Krauss M, Tappe K, Schuppert A, Kuepfer L, Goerlitz L. Bayesian Population Physiologically-Based Pharmacokinetic (PBPK) Approach for a Physiologically Realistic Characterization of Interindividual Variability in Clinically Relevant Populations. PLoS One. 2015; 10:e0139423. DOI: 10.1371/journal.pone.0139423. PMID: 26431198.
Article19. Zhao P, Rowland M, Huang SM. Best practice in the use of physiologically based pharmacokinetic modeling and simulation to address clinical pharmacology regulatory questions. Clin Pharmacol Ther. 2012; 92:17–20. DOI: 10.1038/clpt.2012.68. PMID: 22713733.
Article20. Chun WS. Pharmacokinetics of Cyclosporine A and Its Therapeutic Effect in Children with Renal Diseases. Korean J Pediatr. 2004; 47:193–203.21. Maharaj AR, Barrett JS, Edginton AN. A workflow example of PBPK modeling to support pediatric research and development: case study with lorazepam. AAPS J. 2013; 15:455–464. DOI: 10.1208/s12248-013-9451-0. PMID: 23344790.
Article22. Edginton AN, Schmitt W, Willmann S. Development and evaluation of a generic physiologically based pharmacokinetic model for children. Clin Pharmacokinet. 2006; 45:1013–1034. DOI: 10.2165/00003088-200645100-00005. PMID: 16984214.
Article23. Cremers SC, Scholten EM, Schoemaker RC, Lentjes EG, Vermeij P, Paul LC, et al. A compartmental pharmacokinetic model of cyclosporin and its predictive performance after Bayesian estimation in kidney and simultaneous pancreas-kidney transplant recipients. Nephrol Dial Transplant. 2003; 18:1201–1208. PMID: 12748356.
Article24. Kurose K, Sugiyama E, Saito Y. Population differences in major functional polymorphisms of pharmacokinetics/pharmacodynamics-related genes in Eastern Asians and Europeans: implications in the clinical trials for novel drug development. Drug Metab Pharmacokinet. 2012; 27:9–54. DOI: 10.2133/dmpk.DMPK-11-RV-111. PMID: 22123129.
Article25. Schmidt S, Gonzalez D, Derendorf H. Significance of protein binding in pharmacokinetics and pharmacodynamics. J Pharm Sci. 2010; 99:1107–1122. DOI: 10.1002/jps.21916. PMID: 19852037.
Article26. Sethi PK, White CA, Cummings BS, Hines RN, Muralidhara S, Bruckner JV. Ontogeny of plasma proteins, albumin and binding of diazepam, cyclosporine, and deltamethrin. Pediatr Res. 2016; 79:409–415. DOI: 10.1038/pr.2015.237. PMID: 26571224.
Article27. Rodgers T, Rowland M. Mechanistic approaches to volume of distribution predictions: understanding the processes. Pharm Res. 2007; 24:918–933. DOI: 10.1007/s11095-006-9210-3. PMID: 17372687.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Development of Physiologically Based Pharmacokinetic Model for Several Volatile Organic Compounds
- Compatibility Study between Physiologically Based Pharmacokinetic (PBPK) and Compartmental PK Model Using Lumping Method: Application to the Voriconazole Case
- Evolving role of modeling and simulation in drug development
- Physiological spaces and multicompartmental pharmacokinetic models
- Physiologically-based pharmacokinetic predictions of intestinal BCRP-mediated drug interactions of rosuvastatin in Koreans