J Breast Cancer.
2019 Sep;22(3):412-424. 10.4048/jbc.2019.22.e35.
Potential Benefits of Neoadjuvant Chemotherapy in Clinically Node-Positive Luminal Subtypeâ» Breast Cancer
- Affiliations
-
- 1Division of Breast Surgery, Department of Surgery, Hanyang University Guri Hospital, Hanyang University College of Medicine, Seoul, Korea.
- 2Division of Breast Surgery, Department of Surgery, Seoul St. Mary's Hospital, College of Medicine, The Catholic University of Korea, Seoul, Korea.
- 3Division of Breast Surgery, Department of Surgery, Samsung Medical Center, Sungkyunkwan University School of Medicine, Seoul, Korea. bjchae@gmail.com
- KMID: 2458862
- DOI: http://doi.org/10.4048/jbc.2019.22.e35
Abstract
- PURPOSE
Neoadjuvant chemotherapy (NAC) is less effective for luminal breast cancer because luminal breast cancer has a lower rate of pathological complete response (pCR) after NAC than human epidermal growth factor receptor 2 (HER2)-type and triple-negative breast cancer (TNBC). We investigated the efficacy of NAC and the predictive factors of a better response in luminal breast cancer.
METHODS
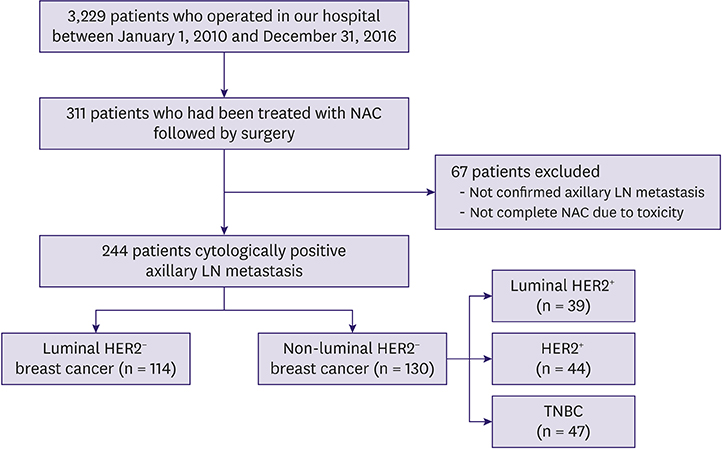
Between 2010 and 2016, we retrieved data of 244 patients with clinically node-positive breast cancer who were treated with NAC followed by surgery from a prospectively collected database. We classified breast cancer into luminal HER2â» and non-luminal HER2â» breast cancer (luminal HER2âº, HER2âº, and TNBC types). We analyzed each subtype with respect to surgical outcomes, response to NAC, and determined variables associated with surgical outcomes and response in patients with luminal HER2â» breast cancer.
RESULTS
The total, breast, and axillary pCR rates were significantly lower in 114 patients with luminal HER2⻠breast cancer than in those with other subtypes (7.9%, 12.3%, and 22.8%, respectively). However, breast-conserving surgery (BCS) conversion and tumor response rates did not significantly differ between patients with luminal HER2⻠and those with non-luminal HER2⻠breast cancer (p = 0.836 and p = 0.180, respectively). In the multivariate analysis, high tumor response rate (≥ 46.4%) was significantly associated with an increased BCS conversion rate. In the subgroup analysis of luminal HER2⻠breast cancer, the multivariate analysis showed that higher Ki67 expression and axilla pCR and BCS conversion rates were significantly associated with tumor response to NAC.
CONCLUSION
Despite the low pCR rate, the tumor response and BCS conversion rates after NAC of luminal HER2â» breast cancer were similar to those of other subtypes. NAC has the potential benefit of reducing the size of breast cancer, thereby increasing the BCS conversion rate in luminal HER2â» breast cancer.
MeSH Terms
Figure
Reference
-
1. Kaufmann M, von Minckwitz G, Smith R, Valero V, Gianni L, Eiermann W, et al. International expert panel on the use of primary (preoperative) systemic treatment of operable breast cancer: review and recommendations. J Clin Oncol. 2003; 21:2600–2608.
Article2. van Nes JG, Putter H, Julien JP, Tubiana-Hulin M, van de Vijver M, Bogaerts J, et al. Preoperative chemotherapy is safe in early breast cancer, even after 10 years of follow-up; clinical and translational results from the EORTC trial 10902. Breast Cancer Res Treat. 2009; 115:101–113.
Article3. Vila J, Mittendorf EA, Farante G, Bassett RL, Veronesi P, Galimberti V, et al. Nomograms for predicting axillary response to neoadjuvant chemotherapy in clinically node-positive patients with breast cancer. Ann Surg Oncol. 2016; 23:3501–3509.
Article4. Fisher B, Brown A, Mamounas E, Wieand S, Robidoux A, Margolese RG, et al. Effect of preoperative chemotherapy on local-regional disease in women with operable breast cancer: findings from National Surgical Adjuvant Breast and Bowel Project B-18. J Clin Oncol. 1997; 15:2483–2493.
Article5. Mauri D, Pavlidis N, Ioannidis JP. Neoadjuvant versus adjuvant systemic treatment in breast cancer: a meta-analysis. J Natl Cancer Inst. 2005; 97:188–194.
Article6. Kuerer HM, Newman LA, Buzdar AU, Hunt KK, Dhingra K, Buchholz TA, et al. Residual metastatic axillary lymph nodes following neoadjuvant chemotherapy predict disease-free survival in patients with locally advanced breast cancer. Am J Surg. 1998; 176:502–509.
Article7. Rastogi P, Anderson SJ, Bear HD, Geyer CE, Kahlenberg MS, Robidoux A, et al. Preoperative chemotherapy: updates of National Surgical Adjuvant Breast and Bowel Project Protocols B-18 and B-27. J Clin Oncol. 2008; 26:778–785.
Article8. Goldhirsch A, Wood WC, Coates AS, Gelber RD, Thürlimann B, Senn HJ, et al. Strategies for subtypes--dealing with the diversity of breast cancer: highlights of the St. Gallen International Expert Consensus on the Primary Therapy of Early Breast Cancer 2011. Ann Oncol. 2011; 22:1736–1747.
Article9. Rouzier R, Perou CM, Symmans WF, Ibrahim N, Cristofanilli M, Anderson K, et al. Breast cancer molecular subtypes respond differently to preoperative chemotherapy. Clin Cancer Res. 2005; 11:5678–5685.
Article10. von Minckwitz G, Untch M, Blohmer JU, Costa SD, Eidtmann H, Fasching PA, et al. Definition and impact of pathologic complete response on prognosis after neoadjuvant chemotherapy in various intrinsic breast cancer subtypes. J Clin Oncol. 2012; 30:1796–1804.
Article11. Boughey JC, McCall LM, Ballman KV, Mittendorf EA, Ahrendt GM, Wilke LG, et al. Tumor biology correlates with rates of breast-conserving surgery and pathologic complete response after neoadjuvant chemotherapy for breast cancer: findings from the ACOSOG Z1071 (Alliance) Prospective Multicenter Clinical Trial. Ann Surg. 2014; 260:608–614.12. Alba E, Calvo L, Albanell J, De la Haba JR, Arcusa Lanza A, Chacon JI, et al. Chemotherapy (CT) and hormonotherapy (HT) as neoadjuvant treatment in luminal breast cancer patients: results from the GEICAM/2006-03, a multicenter, randomized, phase-II study. Ann Oncol. 2012; 23:3069–3074.
Article13. Kim SI, Sohn J, Koo JS, Park SH, Park HS, Park BW. Molecular subtypes and tumor response to neoadjuvant chemotherapy in patients with locally advanced breast cancer. Oncology. 2010; 79:324–330.
Article14. Hammond ME, Hayes DF, Dowsett M, Allred DC, Hagerty KL, Badve S, et al. American Society of Clinical Oncology/College of American Pathologists guideline recommendations for immunohistochemical testing of estrogen and progesterone receptors in breast cancer. Arch Pathol Lab Med. 2010; 134:907–922.
Article15. Liedtke C, Mazouni C, Hess KR, André F, Tordai A, Mejia JA, et al. Response to neoadjuvant therapy and long-term survival in patients with triple-negative breast cancer. J Clin Oncol. 2008; 26:1275–1281.
Article16. Kuerer HM, Sahin AA, Hunt KK, Newman LA, Breslin TM, Ames FC, et al. Incidence and impact of documented eradication of breast cancer axillary lymph node metastases before surgery in patients treated with neoadjuvant chemotherapy. Ann Surg. 1999; 230:72–78.
Article17. Rouzier R, Pusztai L, Delaloge S, Gonzalez-Angulo AM, Andre F, Hess KR, et al. Nomograms to predict pathologic complete response and metastasis-free survival after preoperative chemotherapy for breast cancer. J Clin Oncol. 2005; 23:8331–8339.
Article18. Caudle AS, Yu TK, Tucker SL, Bedrosian I, Litton JK, Gonzalez-Angulo AM, et al. Local-regional control according to surrogate markers of breast cancer subtypes and response to neoadjuvant chemotherapy in breast cancer patients undergoing breast conserving therapy. Breast Cancer Res. 2012; 14:R83.
Article19. Olson JA Jr, Budd GT, Carey LA, Harris LA, Esserman LJ, Fleming GF, et al. Improved surgical outcomes for breast cancer patients receiving neoadjuvant aromatase inhibitor therapy: results from a multicenter phase II trial. J Am Coll Surg. 2009; 208:906–914.
Article20. Barcenas CH, Niu J, Zhang N, Zhang Y, Buchholz TA, Elting LS, et al. Risk of hospitalization according to chemotherapy regimen in early-stage breast cancer. J Clin Oncol. 2014; 32:2010–2017.
Article21. Cortazar P, Zhang L, Untch M, Mehta K, Costantino JP, Wolmark N, et al. Pathological complete response and long-term clinical benefit in breast cancer: the CTNeoBC pooled analysis. Lancet. 2014; 384:164–172.
Article22. Cardoso F, van't Veer LJ, Bogaerts J, Slaets L, Viale G, Delaloge S, et al. 70-gene signature as an aid to treatment decisions in early-stage breast cancer. N Engl J Med. 2016; 375:717–729.
Article23. Park JH, Anderson WF, Gail MH. Improvements in US breast cancer survival and proportion explained by tumor size and estrogen-receptor status. J Clin Oncol. 2015; 33:2870–2876.
Article24. Wang L, Ouyang T, Wang T, Xie Y, Fan Z, Lin B, et al. Breast-conserving therapy and modified radical mastectomy for primary breast carcinoma: a matched comparative study. Chin J Cancer Res. 2015; 27:545–552.25. Chen AM, Meric-Bernstam F, Hunt KK, Thames HD, Oswald MJ, Outlaw ED, et al. Breast conservation after neoadjuvant chemotherapy: the MD Anderson cancer center experience. J Clin Oncol. 2004; 22:2303–2312.
Article26. Boughey JC, Suman VJ, Mittendorf EA, Ahrendt GM, Wilke LG, Taback B, et al. Sentinel lymph node surgery after neoadjuvant chemotherapy in patients with node-positive breast cancer: the ACOSOG Z1071 (Alliance) clinical trial. JAMA. 2013; 310:1455–1461.
Article27. Boileau JF, Poirier B, Basik M, Holloway CM, Gaboury L, Sideris L, et al. Sentinel node biopsy after neoadjuvant chemotherapy in biopsy-proven node-positive breast cancer: the SN FNAC study. J Clin Oncol. 2015; 33:258–264.
Article28. Kang YJ, Han W, Park S, You JY, Yi HW, Park S, et al. Outcome following sentinel lymph node biopsy-guided decisions in breast cancer patients with conversion from positive to negative axillary lymph nodes after neoadjuvant chemotherapy. Breast Cancer Res Treat. 2017; 166:473–480.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Sentinel Lymph Node Biopsy in Breast Cancer: A Clinical Review and Update
- Neoadjuvant Therapy Should Be the Standard of Care for Every Node Positive Breast Cancer Patient
- Sentinel Lymph Node Biopsy in Patients with Clinically Negative Lymph Node After Neoadjuvant Chemotherapy
- Prognostic Factors in Breast Cancer Patients Following Neoadjuvant Chemotherapy
- Prospective Evaluation of the Feasibility of Sentinel Lymph Node Biopsy in Breast Cancer Patients with Negative Axillary Conversion after Neoadjuvant Chemotherapy