Endocrinol Metab.
2019 Sep;34(3):247-262. 10.3803/EnM.2019.34.3.247.
A Review of the Effects of Glucagon-Like Peptide-1 Receptor Agonists and Sodium-Glucose Cotransporter 2 Inhibitors on Lean Body Mass in Humans
- Affiliations
-
- 1Diabetes Research Centre, University of Leicester, Leicester, UK. melanie.davies@uhl-tr.nhs.uk
- 2NIHR Leicester Biomedical Research Centre, University Hospital of Leicester NHS Trust and the University of Leicester, Leicester, UK.
- 3National Centre for Sport and Exercise Medicine, Loughborough University, Loughborough, UK.
- 4NIHR Collaborations for Leadership in Applied Health Research and Care (CLAHRC), Leicester, UK.
- KMID: 2458626
- DOI: http://doi.org/10.3803/EnM.2019.34.3.247
Abstract
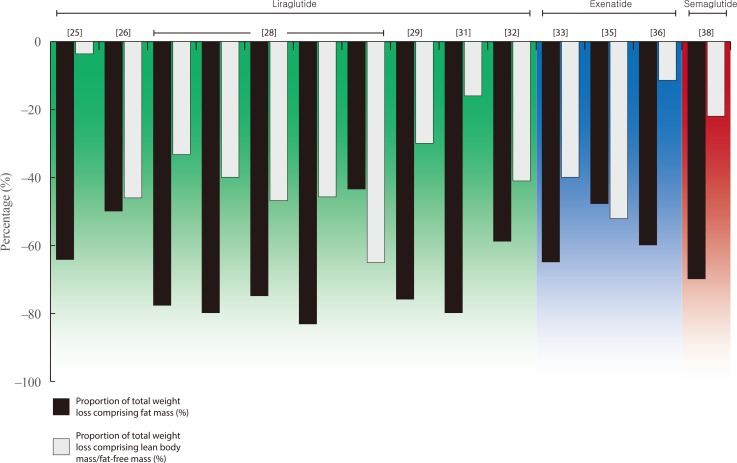
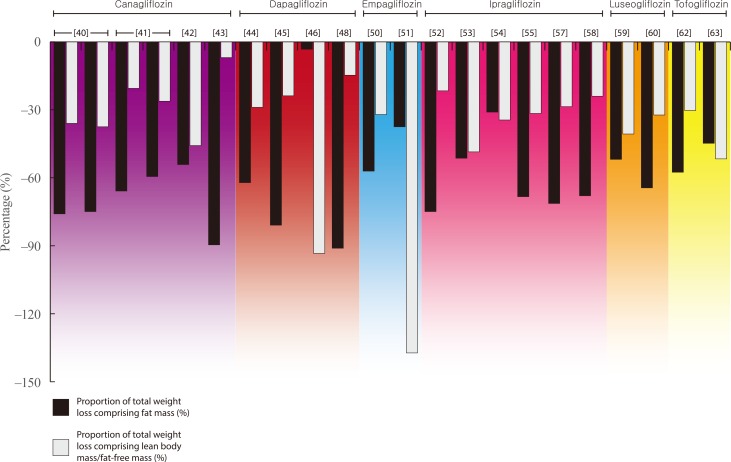
- Weight loss is an important goal in the management of several chronic conditions, including type 2 diabetes mellitus, and pharmacological therapies that aid weight loss are appealing. Glucagon-like peptide-1 receptor agonists (GLP-1RAs) and sodium-glucose cotransporter 2 inhibitors (SGLT2is) are novel glucose-lowering therapies that have been shown to induce clinically significant reductions in body weight. However, this weight loss may not be attributed solely to fat mass (FM). Given the importance of skeletal muscle and lean body mass (LBM) on cardio-metabolic health and physical function, we reviewed the available literature reporting the effects of GLP-1RAs and SGLT2is on body composition. Results demonstrate that, in most circumstances, the weight loss associated with both therapies predominantly comprises a reduction in FM, although significant heterogeneity exists between studies. In over half of the studies identified, the proportion of LBM reduction ranged between 20% and 50% of total weight lost, which is consistent with diet-induced weight loss and bariatric surgery. No clear differences existed between GLP-1RAs and SGLT2is. Consequently, the loss of LBM and skeletal muscle associated with weight loss induced by GLP-1RAs and SGLT2is warrants attention. Strategies to preserve skeletal muscle and improve physical function, for example through structured exercise, are of great importance.
Keyword
MeSH Terms
Figure
Reference
-
1. Davies MJ, D'Alessio DA, Fradkin J, Kernan WN, Mathieu C, Mingrone G, et al. Management of hyperglycaemia in type 2 diabetes, 2018. A consensus report by the American Diabetes Association (ADA) and the European Association for the Study of Diabetes (EASD). Diabetologia. 2018; 61:2461–2498. PMID: 30288571.
Article2. Zelniker TA, Wiviott SD, Raz I, Im K, Goodrich EL, Furtado RHM, et al. Comparison of the effects of glucagon-like peptide receptor agonists and sodium-glucose cotransporter 2 inhibitors for prevention of major adverse cardiovascular and renal outcomes in type 2 diabetes mellitus. Circulation. 2019; 139:2022–2031. PMID: 30786725.
Article3. Gerstein HC, Colhoun HM, Dagenais GR, Diaz R, Lakshmanan M, Pais P, et al. Dulaglutide and cardiovascular outcomes in type 2 diabetes (REWIND): a double-blind, randomized placebo-controlled trial. Lancet. 2019; 394:121–130. PMID: 31189511.4. Zelniker TA, Wiviott SD, Raz I, Im K, Goodrich EL, Bonaca MP, et al. SGLT2 inhibitors for primary and secondary prevention of cardiovascular and renal outcomes in type 2 diabetes: a systematic review and meta-analysis of cardiovascular outcome trials. Lancet. 2019; 393:31–39. PMID: 30424892.
Article5. Perkovic V, Jardine MJ, Neal B, Bompoint S, Heerspink HJL, Charytan DM, et al. Canagliflozin and renal outcomes in type 2 diabetes and nephropathy. N Engl J Med. 2019; 380:2295–2306. PMID: 30990260.
Article6. Pollock C, Stefansson B, Reyner D, Rossing P, Sjostrom CD, Wheeler DC, et al. Albuminuria-lowering effect of dapagliflozin alone and in combination with saxagliptin and effect of dapagliflozin and saxagliptin on glycaemic control in patients with type 2 diabetes and chronic kidney disease (DELIGHT): a randomised, double-blind, placebo-controlled trial. Lancet Diabetes Endocrinol. 2019; 7:429–441. PMID: 30992195.
Article7. Van Gaal L, Scheen A. Weight management in type 2 diabetes: current and emerging approaches to treatment. Diabetes Care. 2015; 38:1161–1172. PMID: 25998297.
Article8. Magkos F, Fraterrigo G, Yoshino J, Luecking C, Kirbach K, Kelly SC, et al. Effects of moderate and subsequent progressive weight loss on metabolic function and adipose tissue biology in humans with obesity. Cell Metab. 2016; 23:591–601. PMID: 26916363.
Article9. Villareal DT, Chode S, Parimi N, Sinacore DR, Hilton T, Armamento-Villareal R, et al. Weight loss, exercise, or both and physical function in obese older adults. N Engl J Med. 2011; 364:1218–1229. PMID: 21449785.
Article10. DeFronzo RA, Tripathy D. Skeletal muscle insulin resistance is the primary defect in type 2 diabetes. Diabetes Care. 2009; 32 Suppl 2:S157–S163. PMID: 19875544.
Article11. Johannsen DL, Knuth ND, Huizenga R, Rood JC, Ravussin E, Hall KD. Metabolic slowing with massive weight loss despite preservation of fat-free mass. J Clin Endocrinol Metab. 2012; 97:2489–2496. PMID: 22535969.
Article12. Cohen S, Nathan JA, Goldberg AL. Muscle wasting in disease: molecular mechanisms and promising therapies. Nat Rev Drug Discov. 2015; 14:58–74. PMID: 25549588.
Article13. McLeod M, Breen L, Hamilton DL, Philp A. Live strong and prosper: the importance of skeletal muscle strength for healthy ageing. Biogerontology. 2016; 17:497–510. PMID: 26791164.
Article14. Sinclair AJ, Abdelhafiz A, Dunning T, Izquierdo M, Rodriguez Manas L, Bourdel-Marchasson I, et al. An international position statement on the management of frailty in diabetes mellitus: summary of recommendations 2017. J Frailty Aging. 2018; 7:10–20. PMID: 29412437.
Article15. Morley JE. Frailty: diagnosis and management. J Nutr Health Aging. 2011; 15:667–670. PMID: 21968862.
Article16. Saum KU, Dieffenbach AK, Muller H, Holleczek B, Hauer K, Brenner H. Frailty prevalence and 10-year survival in community-dwelling older adults: results from the ESTHER cohort study. Eur J Epidemiol. 2014; 29:171–179. PMID: 24671603.
Article17. Morley JE, Malmstrom TK, Rodriguez-Manas L, Sinclair AJ. Frailty, sarcopenia and diabetes. J Am Med Dir Assoc. 2014; 15:853–859. PMID: 25455530.
Article18. Sayer AA, Dennison EM, Syddall HE, Gilbody HJ, Phillips DI, Cooper C. Type 2 diabetes, muscle strength, and impaired physical function: the tip of the iceberg? Diabetes Care. 2005; 28:2541–2542. PMID: 16186295.19. Park SW, Goodpaster BH, Strotmeyer ES, de Rekeneire N, Harris TB, Schwartz AV, et al. Decreased muscle strength and quality in older adults with type 2 diabetes: the health, aging, and body composition study. Diabetes. 2006; 55:1813–1818. PMID: 16731847.20. Delmonico MJ, Harris TB, Visser M, Park SW, Conroy MB, Velasquez-Mieyer P, et al. Longitudinal study of muscle strength, quality, and adipose tissue infiltration. Am J Clin Nutr. 2009; 90:1579–1585. PMID: 19864405.21. Buckinx F, Landi F, Cesari M, Fielding RA, Visser M, Engelke K, et al. Pitfalls in the measurement of muscle mass: a need for a reference standard. J Cachexia Sarcopenia Muscle. 2018; 9:269–278. PMID: 29349935.
Article22. Brozek J, Grande F, Anderson JT, Keys A. Densitometric analysis of body composition: revision of some quantitative assumptions. Ann N Y Acad Sci. 1963; 110:113–140. PMID: 14062375.23. Fields DA, Goran MI, McCrory MA. Body-composition assessment via air-displacement plethysmography in adults and children: a review. Am J Clin Nutr. 2002; 75:453–467. PMID: 11864850.
Article24. Astrup A, Carraro R, Finer N, Harper A, Kunesova M, Lean ME, et al. Safety, tolerability and sustained weight loss over 2 years with the once-daily human GLP-1 analog, liraglutide. Int J Obes (Lond). 2012; 36:843–854. PMID: 21844879.
Article25. Feng WH, Bi Y, Li P, Yin TT, Gao CX, Shen SM, et al. Effects of liraglutide, metformin and gliclazide on body composition in patients with both type 2 diabetes and non-alcoholic fatty liver disease: a randomized trial. J Diabetes Investig. 2019; 10:399–407.
Article26. Frossing S, Nylander M, Chabanova E, Frystyk J, Holst JJ, Kistorp C, et al. Effect of liraglutide on ectopic fat in polycystic ovary syndrome: a randomized clinical trial. Diabetes Obes Metab. 2018; 20:215–218. PMID: 28681988.27. Harder H, Nielsen L, Tu DT, Astrup A. The effect of liraglutide, a long-acting glucagon-like peptide 1 derivative, on glycemic control, body composition, and 24-h energy expenditure in patients with type 2 diabetes. Diabetes Care. 2004; 27:1915–1921. PMID: 15277417.
Article28. Jendle J, Nauck MA, Matthews DR, Frid A, Hermansen K, During M, et al. Weight loss with liraglutide, a once-daily human glucagon-like peptide-1 analogue for type 2 diabetes treatment as monotherapy or added to metformin, is primarily as a result of a reduction in fat tissue. Diabetes Obes Metab. 2009; 11:1163–1172. PMID: 19930006.
Article29. Li CJ, Yu Q, Yu P, Yu TL, Zhang QM, Lu S, et al. Changes in liraglutide-induced body composition are related to modifications in plasma cardiac natriuretic peptides levels in obese type 2 diabetic patients. Cardiovasc Diabetol. 2014; 13:36. PMID: 24498905.
Article30. Perna S, Guido D, Bologna C, Solerte SB, Guerriero F, Isu A, et al. Liraglutide and obesity in elderly: efficacy in fat loss and safety in order to prevent sarcopenia. A perspective case series study. Aging Clin Exp Res. 2016; 28:1251–1257. PMID: 26749118.
Article31. Rondanelli M, Perna S, Astrone P, Grugnetti A, Solerte SB, Guido D. Twenty-four-week effects of liraglutide on body composition, adherence to appetite, and lipid profile in overweight and obese patients with type 2 diabetes mellitus. Patient Prefer Adherence. 2016; 10:407–413. PMID: 27069358.32. Dube MC, D'Amours M, Weisnagel SJ. Beyond glycaemic control: a cross-over, double-blinded, 24-week intervention with liraglutide in type 1 diabetes. Diabetes Obes Metab. 2018; 20:178–184. PMID: 28722271.
Article33. Bradley DP, Kulstad R, Racine N, Shenker Y, Meredith M, Schoeller DA. Alterations in energy balance following exenatide administration. Appl Physiol Nutr Metab. 2012; 37:893–899. PMID: 22735035.
Article34. Bunck MC, Diamant M, Eliasson B, Corner A, Shaginian RM, Heine RJ, et al. Exenatide affects circulating cardiovascular risk biomarkers independently of changes in body composition. Diabetes Care. 2010; 33:1734–1737. PMID: 20424219.
Article35. Ishoy PL, Knop FK, Broberg BV, Bak N, Andersen UB, Jorgensen NR, et al. Effect of GLP-1 receptor agonist treatment on body weight in obese antipsychotic-treated patients with schizophrenia: a randomized, placebo-controlled trial. Diabetes Obes Metab. 2017; 19:162–171. PMID: 27717222.
Article36. Yin TT, Bi Y, Li P, Shen SM, Wang WM, Jiang C, et al. Effects of exenatide versus insulin glargine on body composition in overweight and obese T2DM patients: a randomized controlled trial. Nutr Metab (Lond). 2018; 15:67. PMID: 30302121.
Article37. Hong JY, Park KY, Kim BJ, Hwang WM, Kim DH, Lim DM. Effects of short-term exenatide treatment on regional fat distribution, glycated hemoglobin levels, and aortic pulse wave velocity of obese type 2 diabetes mellitus patients. Endocrinol Metab (Seoul). 2016; 31:80–85. PMID: 26676329.
Article38. Blundell J, Finlayson G, Axelsen M, Flint A, Gibbons C, Kvist T, et al. Effects of once-weekly semaglutide on appetite, energy intake, control of eating, food preference and body weight in subjects with obesity. Diabetes Obes Metab. 2017; 19:1242–1251. PMID: 28266779.
Article39. Seko Y, Sumida Y, Tanaka S, Mori K, Taketani H, Ishiba H, et al. Effect of 12-week dulaglutide therapy in Japanese patients with biopsy-proven non-alcoholic fatty liver disease and type 2 diabetes mellitus. Hepatol Res. 2017; 47:1206–1211. PMID: 27917557.
Article40. Blonde L, Stenlof K, Fung A, Xie J, Canovatchel W, Meininger G. Effects of canagliflozin on body weight and body composition in patients with type 2 diabetes over 104 weeks. Postgrad Med. 2016; 128:371–380. PMID: 27002421.
Article41. Cefalu WT, Leiter LA, Yoon KH, Arias P, Niskanen L, Xie J, et al. Efficacy and safety of canagliflozin versus glimepiride in patients with type 2 diabetes inadequately controlled with metformin (CANTATA-SU): 52 week results from a randomised, double-blind, phase 3 non-inferiority trial. Lancet. 2013; 382:941–950. PMID: 23850055.
Article42. Koike Y, Shirabe SI, Maeda H, Yoshimoto A, Arai K, Kumakura A, et al. Effect of canagliflozin on the overall clinical state including insulin resistance in Japanese patients with type 2 diabetes mellitus. Diabetes Res Clin Pract. 2019; 149:140–146. PMID: 30716347.
Article43. Inoue M, Hayashi A, Taguchi T, Arai R, Sasaki S, Takano K, et al. Effects of canagliflozin on body composition and hepatic fat content in type 2 diabetes patients with non-alcoholic fatty liver disease. J Diabetes Investig. 2019; 10:1004–1011.44. Bolinder J, Ljunggren O, Johansson L, Wilding J, Langkilde AM, Sjostrom CD, et al. Dapagliflozin maintains glycaemic control while reducing weight and body fat mass over 2 years in patients with type 2 diabetes mellitus inadequately controlled on metformin. Diabetes Obes Metab. 2014; 16:159–169. PMID: 23906445.
Article45. Kosugi R, Nakatani E, Okamoto K, Aoshima S, Arai H, Inoue T. Effects of sodium-glucose cotransporter 2 inhibitor (dapagliflozin) on food intake and plasma fibroblast growth factor 21 levels in type 2 diabetes patients. Endocr J. 2019; 66:677–682. PMID: 31130574.
Article46. Fadini GP, Bonora BM, Zatti G, Vitturi N, Iori E, Marescotti MC, et al. Effects of the SGLT2 inhibitor dapagliflozin on HDL cholesterol, particle size, and cholesterol efflux capacity in patients with type 2 diabetes: a randomized placebo-controlled trial. Cardiovasc Diabetol. 2017; 16:42. PMID: 28376855.
Article47. Shimizu M, Suzuki K, Kato K, Jojima T, Iijima T, Murohisa T, et al. Evaluation of the effects of dapagliflozin, a sodium-glucose co-transporter-2 inhibitor, on hepatic steatosis and fibrosis using transient elastography in patients with type 2 diabetes and non-alcoholic fatty liver disease. Diabetes Obes Metab. 2019; 21:285–292. PMID: 30178600.
Article48. Sugiyama S, Jinnouchi H, Kurinami N, Hieshima K, Yoshida A, Jinnouchi K, et al. Dapagliflozin reduces fat mass without affecting muscle mass in type 2 diabetes. J Atheroscler Thromb. 2018; 25:467–476. PMID: 29225209.
Article49. Tobita H, Sato S, Miyake T, Ishihara S, Kinoshita Y. Effects of dapagliflozin on body composition and liver tests in patients with nonalcoholic steatohepatitis associated with type 2 diabetes mellitus: a prospective, open-label, uncontrolled study. Curr Ther Res Clin Exp. 2017; 87:13–19. PMID: 28912902.
Article50. Ridderstrale M, Andersen KR, Zeller C, Kim G, Woerle HJ, Broedl UC, et al. Comparison of empagliflozin and glimepiride as add-on to metformin in patients with type 2 diabetes: a 104-week randomised, active-controlled, double-blind, phase 3 trial. Lancet Diabetes Endocrinol. 2014; 2:691–700. PMID: 24948511.51. Javed Z, Papageorgiou M, Deshmukh H, Rigby AS, Qamar U, Abbas J, et al. Effects of empagliflozin on metabolic parameters in polycystic ovary syndrome: a randomized controlled study. Clin Endocrinol (Oxf). 2019; 90:805–813. PMID: 30866088.
Article52. Inoue H, Morino K, Ugi S, Tanaka-Mizuno S, Fuse K, Miyazawa I, et al. Ipragliflozin, a sodium-glucose cotransporter 2 inhibitor, reduces bodyweight and fat mass, but not muscle mass, in Japanese type 2 diabetes patients treated with insulin: a randomized clinical trial. J Diabetes Investig. 2019; 10:1012–1021.
Article53. Ohta A, Kato H, Ishii S, Sasaki Y, Nakamura Y, Nakagawa T, et al. Ipragliflozin, a sodium glucose co-transporter 2 inhibitor, reduces intrahepatic lipid content and abdominal visceral fat volume in patients with type 2 diabetes. Expert Opin Pharmacother. 2017; 18:1433–1438. PMID: 28770629.
Article54. Iemitsu K, Kawata T, Iizuka T, Takihata M, Takai M, Nakajima S, et al. Efficacy and safety of ipragliflozin in patients with type 2 diabetes: ASSIGN-K study. J Endocrinol Metab. 2019; 9:51–62.
Article55. Kato M, Sakai K, Saito K, Tsutsui K, Yamashita S, Kato N. Efficacy and safety of ipragliflozin in Japanese patients with type 2 diabetes receiving conventional therapy: clinical implication of the importance of exercise habits during treatment with ipragliflozin. Diabetol Int. 2017; 8:275–285. PMID: 30603333.
Article56. Miyake T, Yoshida S, Furukawa S, Sakai T, Tada F, Senba H, et al. Ipragliflozin ameliorates liver damage in non-alcoholic fatty liver disease. . Open Med (Wars). 2018; 13:402–409. PMID: 30234161.
Article57. Osonoi T, Nakamoto S, Saito M, Tamasawa A, Ishida H, Osonoi Y. Efficacy of ipragliflozin as monotherapy or as add-on therapy with other oral antidiabetic medications for treating type 2 diabetes in Japanese patients with inadequate glycemic control: a subgroup analysis based on patient characteristics. J Diabetes Investig. 2018; 9:341–353.58. Yamamoto C, Miyoshi H, Ono K, Sugawara H, Kameda R, Ichiyama M, et al. Ipragliflozin effectively reduced visceral fat in Japanese patients with type 2 diabetes under adequate diet therapy. Endocr J. 2016; 63:589–596. PMID: 27052123.
Article59. Bouchi R, Terashima M, Sasahara Y, Asakawa M, Fukuda T, Takeuchi T, et al. Luseogliflozin reduces epicardial fat accumulation in patients with type 2 diabetes: a pilot study. Cardiovasc Diabetol. 2017; 16:32. PMID: 28253918.
Article60. Sasaki T, Sugawara M, Fukuda M. Sodium-glucose cotransporter 2 inhibitor-induced changes in body composition and simultaneous changes in metabolic profile: 52-week prospective LIGHT (Luseogliflozin: the Components of Weight Loss in Japanese Patients with Type 2 Diabetes Mellitus) Study. J Diabetes Investig. 2019; 10:108–117.
Article61. Iwahashi Y, Hirose S, Nakajima S, Seo A, Takahashi T, Tamori Y. Evaluation of metabolic parameters and body composition in Japanese patients with type 2 diabetes mellitus who were administered tofogliflozin for 48 weeks. Diabetol Int. 2016; 8:205–211. PMID: 30603323.
Article62. Kamei S, Iwamoto M, Kameyama M, Shimoda M, Kinoshita T, Obata A, et al. Effect of tofogliflozin on body composition and glycemic control in Japanese subjects with type 2 diabetes mellitus. J Diabetes Res. 2018; 2018:6470137. PMID: 29507863.
Article63. Matsuba R, Matsuba I, Shimokawa M, Nagai Y, Tanaka Y. Tofogliflozin decreases body fat mass and improves peripheral insulin resistance. Diabetes Obes Metab. 2018; 20:1311–1315. PMID: 29316197.
Article64. Arase Y, Shiraishi K, Anzai K, Sato H, Teramura E, Tsuruya K, et al. Effect of sodium glucose co-transporter 2 inhibitors on liver fat mass and body composition in patients with nonalcoholic fatty liver disease and type 2 diabetes mellitus. Clin Drug Investig. 2019; 39:631–641.65. Kinoshita T, Shimoda M, Sanada J, Fushimi Y, Hirata Y, Irie S, et al. There is a close association between the recovery of liver injury and glycemic control after SGLT2 inhibitor treatment in Japanese subjects with type 2 diabetes: a retrospective clinical study. Diabetes Ther. 2018; 9:1569–1580. PMID: 29931506.
Article66. Seko Y, Sumida Y, Tanaka S, Mori K, Taketani H, Ishiba H, et al. Effect of sodium glucose cotransporter 2 inhibitor on liver function tests in Japanese patients with non-alcoholic fatty liver disease and type 2 diabetes mellitus. Hepatol Res. 2017; 47:1072–1078. PMID: 27925353.
Article67. Lundkvist P, Pereira MJ, Katsogiannos P, Sjostrom CD, Johnsson E, Eriksson JW. Dapagliflozin once daily plus exenatide once weekly in obese adults without diabetes: sustained reductions in body weight, glycaemia and blood pressure over 1 year. Diabetes Obes Metab. 2017; 19:1276–1288. PMID: 28345814.68. Seino Y, Yabe D, Sasaki T, Fukatsu A, Imazeki H, Ochiai H, et al. Sodium-glucose cotransporter-2 inhibitor luseogliflozin added to glucagon-like peptide 1 receptor agonist liraglutide improves glycemic control with bodyweight and fat mass reductions in Japanese patients with type 2 diabetes: a 52-week, open-label, single-arm study. J Diabetes Investig. 2018; 9:332–340.69. Schneider J, Peterli R, Gass M, Slawik M, Peters T, Wolnerhanssen BK. Laparoscopic sleeve gastrectomy and Roux-en-Y gastric bypass lead to equal changes in body composition and energy metabolism 17 months postoperatively: a prospective randomized trial. Surg Obes Relat Dis. 2016; 12:563–570. PMID: 26656669.
Article70. Davidson LE, Yu W, Goodpaster BH, DeLany JP, Widen E, Lemos T, et al. Fat-free mass and skeletal muscle mass five years after bariatric surgery. Obesity (Silver Spring). 2018; 26:1130–1136. PMID: 29845744.
Article71. Chaston TB, Dixon JB, O'Brien PE. Changes in fat-free mass during significant weight loss: a systematic review. Int J Obes (Lond). 2007; 31:743–750. PMID: 17075583.
Article72. Lean ME, Leslie WS, Barnes AC, Brosnahan N, Thom G, McCombie L, et al. Primary care-led weight management for remission of type 2 diabetes (DiRECT): an open-label, cluster-randomised trial. Lancet. 2018; 391:541–551. PMID: 29221645.
Article73. Sjostrom L, Peltonen M, Jacobson P, Ahlin S, Andersson-Assarsson J, Anveden A, et al. Association of bariatric surgery with long-term remission of type 2 diabetes and with microvascular and macrovascular complications. JAMA. 2014; 311:2297–2304. PMID: 24915261.
Article74. Heymsfield SB, Gonzalez MC, Shen W, Redman L, Thomas D. Weight loss composition is one-fourth fat-free mass: a critical review and critique of this widely cited rule. Obes Rev. 2014; 15:310–321. PMID: 24447775.
Article75. Janssen I, Heymsfield SB, Ross R. Low relative skeletal muscle mass (sarcopenia) in older persons is associated with functional impairment and physical disability. J Am Geriatr Soc. 2002; 50:889–896. PMID: 12028177.
Article76. Santanasto AJ, Glynn NW, Newman MA, Taylor CA, Brooks MM, Goodpaster BH, et al. Impact of weight loss on physical function with changes in strength, muscle mass, and muscle fat infiltration in overweight to moderately obese older adults: a randomized clinical trial. J Obes. 2011; 2011:516576. PMID: 20953373.
Article77. Nicklas BJ, Chmelo E, Delbono O, Carr JJ, Lyles MF, Marsh AP. Effects of resistance training with and without caloric restriction on physical function and mobility in overweight and obese older adults: a randomized controlled trial. Am J Clin Nutr. 2015; 101:991–999. PMID: 25762810.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- The Effects of Hypoglycemic Agents on Non-alcoholic Fatty Liver Disease: Focused on Sodium-Glucose Cotransporter 2 Inhibitors and Glucagon-Like Peptide-1 Receptor Agonists
- New anti-diabetic agents
- Characteristics of the Latest Therapeutic Agent for Diabetes
- The Role of Glucagon-Like Peptide 1 Receptor Agonists and Sodium-Glucose Cotransporter 2 Inhibitors in Reducing Cardiovascular Events in Patients with Type 2 Diabetes
- Safety Issues with Sodium-Glucose Cotransporter 2 Inhibitors: Clinical Considerations