Chonnam Med J.
2018 Jan;54(1):24-30. 10.4068/cmj.2018.54.1.24.
Antidiabetic Drug Metformin Protects Neuronal Cells against Quinolinic Acid-Induced Excitotoxicity by Decreasing Intracellular Calcium
- Affiliations
-
- 1Department of Physiology, Chonnam National University Medical School, Gwangju, Korea. hoonpark@jnu.ac.kr
- 2Research Institute of Medical Sciences, Chonnam National University, Gwangju, Korea.
- KMID: 2458581
- DOI: http://doi.org/10.4068/cmj.2018.54.1.24
Abstract
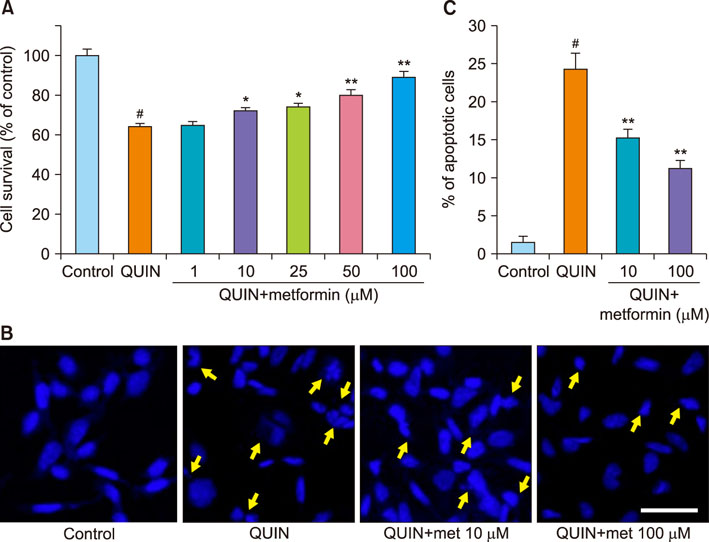
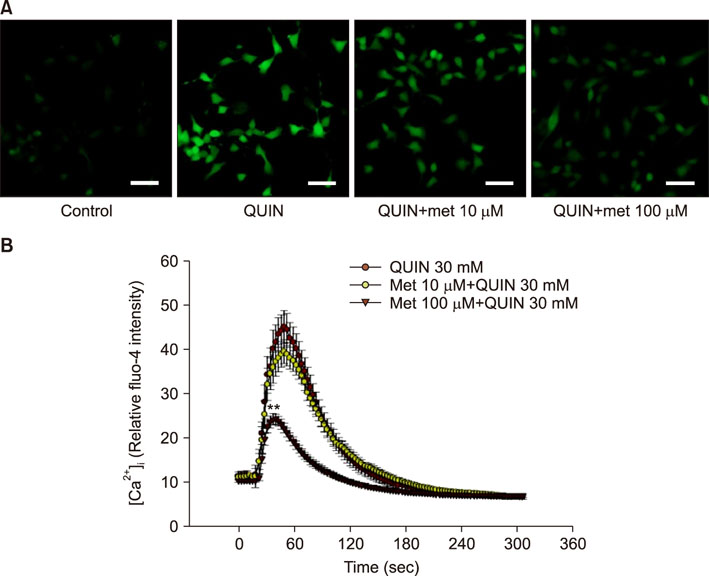
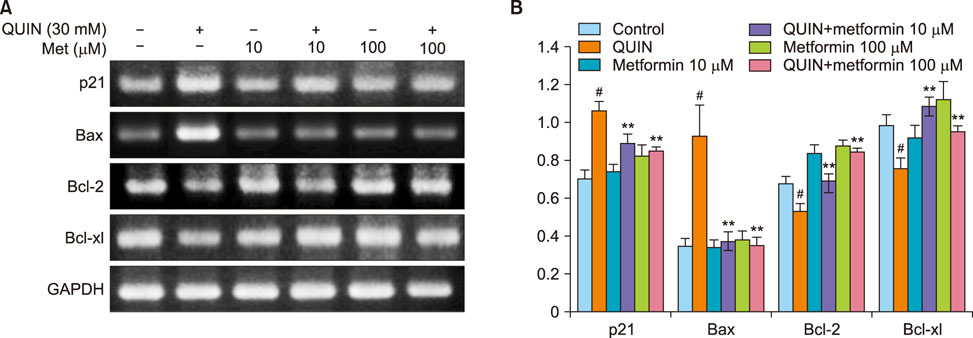
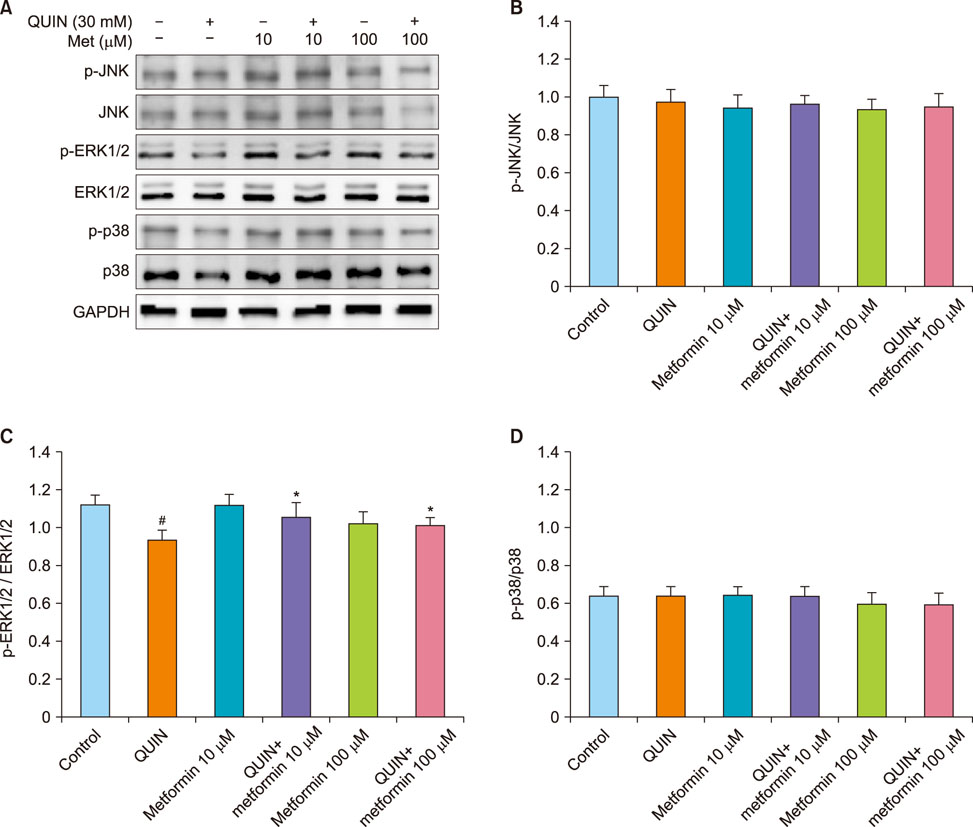
- The antidiabetic drug metformin has been found to have beneficial effects in various neurological disorders; however, the molecular mechanisms underlying these effects remain unclear. Here we report that metformin protects neuronal cells from quinolinic acid (QUIN)-induced excitotoxicity. For this, we pretreated N18D3 neuronal cells with metformin prior to QUIN for 24 h. We found that pretreating the cells with metformin significantly improved cell survival rate in a concentration-dependent manner and reduced apoptotic cell death, as revealed by a MTT assay and DAPI staining, respectively. Calcium imaging using fluo-4 showed that metformin (100 µM) inhibited the intracellular calcium increase that was induced by QUIN. In addition, mRNA expression of pro-apoptotic genes, p21 and Bax, was decreased and of anti-apoptotic genes, Bcl-2 and Bcl-xl, was increased with metformin treatment compared to QUIN-induced cells. The immunoreactivity of phosphorylated ERK1/2 was elevated in cells treated with metformin, indicating the ERK1/2 signaling pathway in the neuroprotective effects of metformin in QUIN-induced cell death. Collectively, our data demonstrates that metformin exerts its neuroprotective effects by inhibiting intracellular calcium increases, allowing it to regulate ERK1/2 signaling and modulate cell survival and death genes.
Keyword
MeSH Terms
Figure
Reference
-
1. Busse M, Hettler V, Fischer V, Mawrin C, Hartig R, Dobrowolny H, et al. Increased quinolinic acid in peripheral mononuclear cells in Alzheimer's dementia. Eur Arch Psychiatry Clin Neurosci. 2017; DOI: 10.1007/s00406-017-0785-y. [Epub ahead of print].
Article2. Breda C, Sathyasaikumar KV, Sograte Idrissi S, Notarangelo FM, Estranero JG, Moore GG, et al. Tryptophan-2,3-dioxygenase (TDO) inhibition ameliorates neurodegeneration by modulation of kynurenine pathway metabolites. Proc Natl Acad Sci U S A. 2016; 113:5435–5440.
Article3. Lugo-Huitrón R, Ugalde Muñiz P, Pineda B, Pedraza-Chaverrí J, Ríos C, Pérez-de la. Quinolinic acid: an endogenous neurotoxin with multiple targets. Oxid Med Cell Longev. 2013; 2013:104024.
Article4. Guillemin GJ. Quinolinic acid, the inescapable neurotoxin. FEBS J. 2012; 279:1356–1365.
Article5. Kritis AA, Stamoula EG, Paniskaki KA, Vavilis TD. Researching glutamate - induced cytotoxicity in different cell lines: a comparative/ collective analysis/study. Front Cell Neurosci. 2015; 9:91.
Article6. Jang S, Jeong HS, Park JS, Kim YS, Jin CY, Seol MB, et al. Neuroprotective effects of (-)-epigallocatechin-3-gallate against quinolinic acid-induced excitotoxicity via PI3K pathway and NO inhibition. Brain Res. 2010; 1313:25–33.
Article7. Latif-Hernandez A, Shah D, Ahmed T, Lo AC, Callaerts-Vegh Z, Van der Linden A, et al. Quinolinic acid injection in mouse medial prefrontal cortex affects reversal learning abilities, cortical connectivity and hippocampal synaptic plasticity. Sci Rep. 2016; 6:36489.
Article8. Mishra J, Chaudhary T, Kumar A. Rosiglitazone synergizes the neuroprotective effects of valproic acid against quinolinic acid-induced neurotoxicity in rats: targeting PPARγ and HDAC pathways. Neurotox Res. 2014; 26:130–151.
Article9. Jiang T, Yu JT, Zhu XC, Wang HF, Tan MS, Cao L, et al. Acute metformin preconditioning confers neuroprotection against focal cerebral ischaemia by pre-activation of AMPK-dependent autophagy. Br J Pharmacol. 2014; 171:3146–3157.
Article10. Venna VR, Li J, Hammond MD, Mancini NS, McCullough LD. Chronic metformin treatment improves post-stroke angiogenesis and recovery after experimental stroke. Eur J Neurosci. 2014; 39:2129–2138.
Article11. Ge XH, Zhu GJ, Geng DQ, Zhang HZ, He JM, Guo AZ, et al. Metformin protects the brain against ischemia/reperfusion injury through PI3K/Akt1/JNK3 signaling pathways in rats. Physiol Behav. 2017; 170:115–123.
Article12. Wang C, Liu C, Gao K, Zhao H, Zhou Z, Shen Z, et al. Metformin preconditioning provide neuroprotection through enhancement of autophagy and suppression of inflammation and apoptosis after spinal cord injury. Biochem Biophys Res Commun. 2016; 477:534–540.
Article13. Chang J, Jung HH, Yang JY, Lee S, Choi J, Im GJ, et al. Protective effect of metformin against cisplatin-induced ototoxicity in an auditory cell line. J Assoc Res Otolaryngol. 2014; 15:149–158.
Article14. Ismaiel AA, Espinosa-Oliva AM, Santiago M, García-Quintanilla A, Oliva-Martín MJ, Herrera AJ, et al. Metformin, besides exhibiting strong in vivo anti-inflammatory properties, increases mptp-induced damage to the nigrostriatal dopaminergic system. Toxicol Appl Pharmacol. 2016; 298:19–30.
Article15. Chen Y, Zhou K, Wang R, Liu Y, Kwak YD, Ma T, et al. Antidiabetic drug metformin (GlucophageR) increases biogenesis of Alzheimer's amyloid peptides via up-regulating BACE1 transcription. Proc Natl Acad Sci U S A. 2009; 106:3907–3912.
Article16. Gupta A, Bisht B, Dey CS. Peripheral insulin-sensitizer drug metformin ameliorates neuronal insulin resistance and Alzheimer's-like changes. Neuropharmacology. 2011; 60:910–920.
Article17. Asadbegi M, Yaghmaei P, Salehi I, Ebrahim-Habibi A, Komaki A. Neuroprotective effects of metformin against Aβ-mediated inhibition of long-term potentiation in rats fed a high-fat diet. Brain Res Bull. 2016; 121:178–185.
Article18. Jang S, Park JS, Jeong HS. Neural differentiation of human adipose tissue-derived stem cells involves activation of the Wnt5a/JNK signalling. Stem Cells Int. 2015; 2015:178618.
Article19. Xia Z, Dickens M, Raingeaud J, Davis RJ, Greenberg ME. Opposing effects of ERK and JNK-p38 MAP kinases on apoptosis. Science. 1995; 270:1326–1331.
Article20. Lu Z, Xu S. ERK1/2 MAP kinases in cell survival and apoptosis. IUBMB Life. 2006; 58:621–631.
Article21. Zhou C, Sun R, Zhuang S, Sun C, Jiang Y, Cui Y, et al. Metformin prevents cerebellar granule neurons against glutamate-induced neurotoxicity. Brain Res Bull. 2016; 121:241–245.
Article22. Khallaghi B, Safarian F, Nasoohi S, Ahmadiani A, Dargahi L. Metformin-induced protection against oxidative stress is associated with AKT/mTOR restoration in PC12 cells. Life Sci. 2016; 148:286–292.
Article23. Chang J, Jung HH, Yang JY, Choi J, Im GJ, Chae SW. Protective role of antidiabetic drug metformin against gentamicin induced apoptosis in auditory cell line. Hear Res. 2011; 282:92–96.
Article24. Ullah I, Ullah N, Naseer MI, Lee HY, Kim MO. Neuroprotection with metformin and thymoquinone against ethanol-induced apoptotic neurodegeneration in prenatal rat cortical neurons. BMC Neurosci. 2012; 13:11.
Article25. Rahman A, Ting K, Cullen KM, Braidy N, Brew BJ, Guillemin GJ. The excitotoxin quinolinic acid induces tau phosphorylation in human neurons. PLoS One. 2009; 4:e6344.
Article26. Lai TW, Zhang S, Wang YT. Excitotoxicity and stroke: identifying novel targets for neuroprotection. Prog Neurobiol. 2014; 115:157–188.
Article27. Chen B, Teng Y, Zhang X, Lv X, Yin Y. Metformin alleviated Aβ-induced apoptosis via the suppression of JNK MAPK signaling pathway in cultured hippocampal neurons. Biomed Res Int. 2016; 2016:1421430.
Article28. Li Q, Chen M, Liu H, Yang L, Yang T, He G. The dual role of ERK signaling in the apoptosis of neurons. Front Biosci (Landmark Ed). 2014; 19:1411–1417.
Article29. Fang XX, Jiang XL, Han XH, Peng YP, Qiu YH. Neuroprotection of interleukin-6 against NMDA-induced neurotoxicity is mediated by JAK/STAT3, MAPK/ERK, and PI3K/AKT signaling pathways. Cell Mol Neurobiol. 2013; 33:241–251.
Article30. Hu S, Cui W, Mak S, Tang J, Choi C, Pang Y, et al. Bis(propyl)-cognitin protects against glutamate-induced neuro-excitotoxicity via concurrent regulation of NO, MAPK/ERK and PI3-K/Akt/GSK3β pathways. Neurochem Int. 2013; 62:468–477.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Protective Effect of Metformin on Gentamicin-Induced Vestibulotoxicity in Rat Primary Cell Culture
- 4-hydroxy-2(E)-Nonenal facilitates NMDA-Induced Neurotoxicity via Triggering Mitochondrial Permeability Transition Pore Opening and Mitochondrial Calcium Overload
- Effects of the Immunoglobulins of Amyotrophic Lateral Sclerosis on Intracellular Calcium in PC12 Cells
- Propofol and Aminophylline Antagonize Each Other During the Mobilization of Intracellular Calcium in Human Umbilical Vein Endothelial Cells
- Preventive effects of imperatorin on perfluorohexanesulfonate-induced neuronal apoptosis via inhibition of intracellular calcium-mediated ERK pathway