Korean J Radiol.
2016 Oct;17(5):779-788. 10.3348/kjr.2016.17.5.779.
Therapeutic Effects of Microbubbles Added to Combined High-Intensity Focused Ultrasound and Chemotherapy in a Pancreatic Cancer Xenograft Model
- Affiliations
-
- 1Department of Radiology, Konkuk University Medical Center, Seoul 05030, Korea.
- 2Department of Radiology, Seoul National University Hospital, Seoul 03080, Korea. leejy4u@snu.ac.kr
- 3Department of Pre-Dentistry, Gangneung-Wonju National University College of Dentistry, Gangneung 25457, Korea.
- 4Department of Radiology, Chung-Ang University Hospital, Seoul 06973, Korea.
- KMID: 2458071
- DOI: http://doi.org/10.3348/kjr.2016.17.5.779
Abstract
OBJECTIVE
To investigate whether high-intensity focused ultrasound (HIFU) combined with microbubbles enhances the therapeutic effects of chemotherapy.
MATERIALS AND METHODS
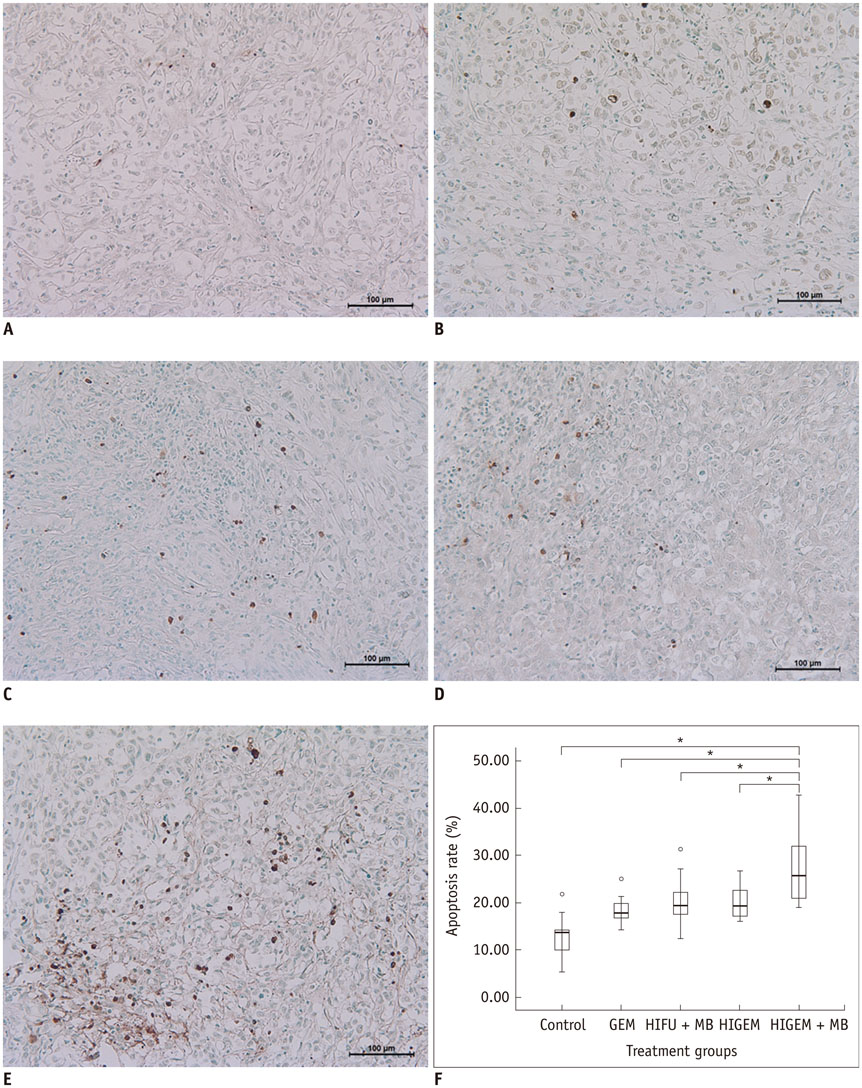
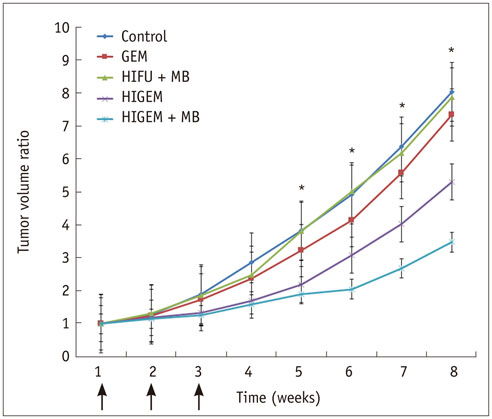
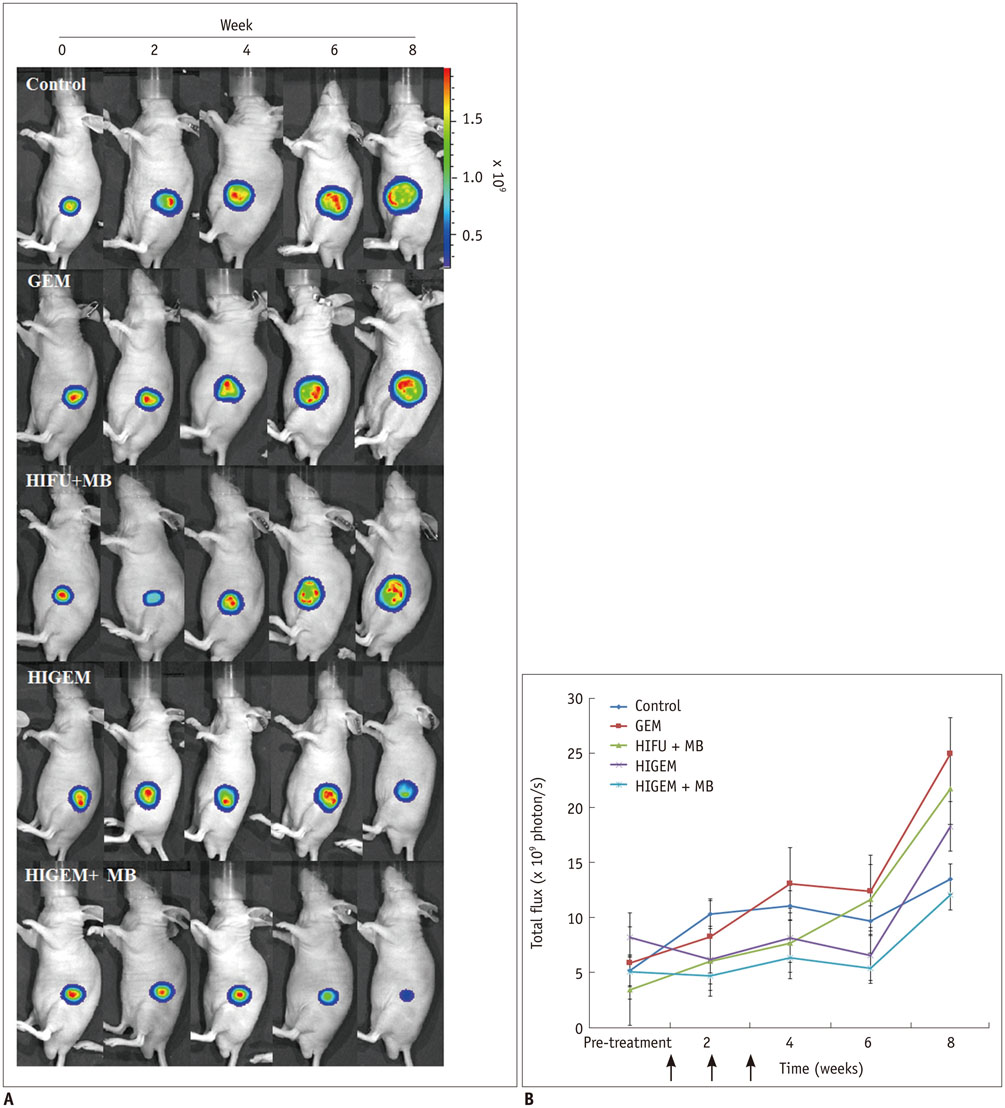
A pancreatic cancer xenograft model was established using BALB/c nude mice and luciferase-expressing human pancreatic cancer cells. Mice were randomly assigned to five groups according to treatment: control (n = 10), gemcitabine alone (GEM; n = 12), HIFU with microbubbles (HIFU + MB, n = 11), combined HIFU and gemcitabine (HIGEM; n = 12), and HIGEM + MB (n = 13). After three weekly treatments, apoptosis rates were evaluated using the terminal deoxynucleotidyl transferase-mediated dUTP nick end-labeling assay in two mice per group. Tumor volume and bioluminescence were monitored using high-resolution 3D ultrasound imaging and in vivo bioluminescence imaging for eight weeks in the remaining mice.
RESULTS
The HIGEM + MB group showed significantly higher apoptosis rates than the other groups (p < 0.05) and exhibited the slowest tumor growth. From week 5, the tumor-volume-ratio relative to the baseline tumor volume was significantly lower in the HIGEM + MB group than in the control, GEM, and HIFU + MB groups (p < 0.05). Despite visible distinction, the HIGEM and HIGEM + MB groups showed no significant differences.
CONCLUSION
High-intensity focused ultrasound combined with microbubbles enhances the therapeutic effects of gemcitabine chemotherapy in a pancreatic cancer xenograft model.
Keyword
MeSH Terms
-
Animals
Antimetabolites, Antineoplastic/*therapeutic use
Apoptosis
Cell Line, Tumor
Combined Modality Therapy
Deoxycytidine/*analogs & derivatives/therapeutic use
Humans
Mice, Inbred BALB C
Mice, Nude
Microbubbles/*therapeutic use
Pancreatic Neoplasms/diagnostic imaging/pathology/*therapy
Tumor Burden
Ultrasonic Therapy/*methods
Ultrasonography
Xenograft Model Antitumor Assays
Antimetabolites, Antineoplastic
Deoxycytidine
Figure
Reference
-
1. von Wichert G, Seufferlein T, Adler G. Palliative treatment of pancreatic cancer. J Dig Dis. 2008; 9:1–7.2. Hariharan D, Saied A, Kocher HM. Analysis of mortality rates for pancreatic cancer across the world. HPB (Oxford). 2008; 10:58–56.3. Cardenes HR, Chiorean EG, Dewitt J, Schmidt M, Loehrer P. Locally advanced pancreatic cancer: current therapeutic approach. Oncologist. 2006; 11:612–623.4. Maréchal R, Bachet JB, Mackey JR, Dalban C, Demetter P, Graham K, et al. Levels of gemcitabine transport and metabolism proteins predict survival times of patients treated with gemcitabine for pancreatic adenocarcinoma. Gastroenterology. 2012; 143:664–674.5. el-Kamar FG, Grossbard ML, Kozuch PS. Metastatic pancreatic cancer: emerging strategies in chemotherapy and palliative care. Oncologist. 2003; 8:18–34.6. Burris HA 3rd, Moore MJ, Andersen J, Green MR, Rothenberg ML, Modiano MR, et al. Improvements in survival and clinical benefit with gemcitabine as first-line therapy for patients with advanced pancreas cancer: a randomized trial. J Clin Oncol. 1997; 15:2403–2413.7. Mukherjee S, Hudson E, Reza S, Thomas M, Crosby T, Maughan T. Pancreatic cancer within a UK cancer network with special emphasis on locally advanced non-metastatic pancreatic cancer. Clin Oncol (R Coll Radiol). 2008; 20:535–554.8. Orsi F, Arnone P, Chen W, Zhang L. High intensity focused ultrasound ablation: a new therapeutic option for solid tumors. J Cancer Res Ther. 2010; 6:414–420.9. Jang HJ, Lee JY, Lee DH, Kim WH, Hwang JH. Current and future clinical applications of high-intensity focused ultrasound (HIFU) for pancreatic cancer. Gut Liver. 2010; 4:Suppl 1. S57–S61.10. Wu F, Wang ZB, Zhu H, Chen WZ, Zou JZ, Bai J, et al. Feasibility of US-guided high-intensity focused ultrasound treatment in patients with advanced pancreatic cancer: initial experience. Radiology. 2005; 236:1034–1040.11. Xiong LL, Hwang JH, Huang XB, Yao SS, He CJ, Ge XH, et al. Early clinical experience using high intensity focused ultrasound for palliation of inoperable pancreatic cancer. JOP. 2009; 10:123–129.12. Sofuni A, Moriyasu F, Sano T, Yamada K, Itokawa F, Tsuchiya T, et al. The current potential of high-intensity focused ultrasound for pancreatic carcinoma. J Hepatobiliary Pancreat Sci. 2011; 18:295–303.13. Sung HY, Jung SE, Cho SH, Zhou K, Han JY, Han ST, et al. Long-term outcome of high-intensity focused ultrasound in advanced pancreatic cancer. Pancreas. 2011; 40:1080–1086.14. Li PZ, Zhu SH, He W, Zhu LY, Liu SP, Liu Y, et al. High-intensity focused ultrasound treatment for patients with unresectable pancreatic cancer. Hepatobiliary Pancreat Dis Int. 2012; 11:655–660.15. Zhao H, Yang G, Wang D, Yu X, Zhang Y, Zhu J, et al. Concurrent gemcitabine and high-intensity focused ultrasound therapy in patients with locally advanced pancreatic cancer. Anticancer Drugs. 2010; 21:447–452.16. Lee JY, Choi BI, Ryu JK, Kim YT, Hwang JH, Kim SH, et al. Concurrent chemotherapy and pulsed high-intensity focused ultrasound therapy for the treatment of unresectable pancreatic cancer: initial experiences. Korean J Radiol. 2011; 12:176–186.17. Iwanaga K, Tominaga K, Yamamoto K, Habu M, Maeda H, Akifusa S, et al. Local delivery system of cytotoxic agents to tumors by focused sonoporation. Cancer Gene Ther. 2007; 14:354–363.18. Karshafian R, Bevan PD, Williams R, Samac S, Burns PN. Sonoporation by ultrasound-activated microbubble contrast agents: effect of acoustic exposure parameters on cell membrane permeability and cell viability. Ultrasound Med Biol. 2009; 35:847–860.19. Bazan-Peregrino M, Arvanitis CD, Rifai B, Seymour LW, Coussios CC. Ultrasound-induced cavitation enhances the delivery and therapeutic efficacy of an oncolytic virus in an in vitro model. J Control Release. 2012; 157:235–242.20. Kudo N, Okada K, Yamamoto K. Sonoporation by single-shot pulsed ultrasound with microbubbles adjacent to cells. Biophys J. 2009; 96:4866–4876.21. Tzu-Yin W, Wilson KE, Machtaler S, Willmann JK. Ultrasound and microbubble guided drug delivery: mechanistic understanding and clinical implications. Curr Pharm Biotechnol. 2013; 14:743–752.22. Delalande A, Kotopoulis S, Postema M, Midoux P, Pichon C. Sonoporation: mechanistic insights and ongoing challenges for gene transfer. Gene. 2013; 525:191–199.23. Ibsen S, Schutt CE, Esener S. Microbubble-mediated ultrasound therapy: a review of its potential in cancer treatment. Drug Des Devel Ther. 2013; 7:375–388.24. Lawrie A, Brisken AF, Francis SE, Tayler DI, Chamberlain J, Crossman DC, et al. Ultrasound enhances reporter gene expression after transfection of vascular cells in vitro. Circulation. 1999; 99:2617–2620.25. Lindner JR. Microbubbles in medical imaging: current applications and future directions. Nat Rev Drug Discov. 2004; 3:527–532.26. Hernot S, Klibanov AL. Microbubbles in ultrasound-triggered drug and gene delivery. Adv Drug Deliv Rev. 2008; 60:1153–1166.27. Suzuki R, Oda Y, Utoguchi N, Maruyama K. Progress in the development of ultrasound-mediated gene delivery systems utilizing nano- and microbubbles. J Control Release. 2011; 149:36–41.28. Watanabe Y, Aoi A, Horie S, Tomita N, Mori S, Morikawa H, et al. Low-intensity ultrasound and microbubbles enhance the antitumor effect of cisplatin. Cancer Sci. 2008; 99:2525–2531.29. Lee NG, Berry JL, Lee TC, Wang AT, Honowitz S, Murphree AL, et al. Sonoporation enhances chemotherapeutic efficacy in retinoblastoma cells in vitro. Invest Ophthalmol Vis Sci. 2011; 52:3868–3873.30. Kotopoulis S, Dimcevski G, Gilja OH, Hoem D, Postema M. Treatment of human pancreatic cancer using combined ultrasound, microbubbles, and gemcitabine: a clinical case study. Med Phys. 2013; 40:072902.31. Kotopoulis S, Delalande A, Popa M, Mamaeva V, Dimcevski G, Gilja OH, et al. Sonoporation-enhanced chemotherapy significantly reduces primary tumour burden in an orthotopic pancreatic cancer xenograft. Mol Imaging Biol. 2014; 16:53–62.32. Kim JH, Kim H, Kim YJ, Lee JY, Han JK, Choi BI. Dynamic contrast-enhanced ultrasonographic (DCE-US) assessment of the early response after combined gemcitabine and HIFU with low-power treatment for the mouse xenograft model of human pancreatic cancer. Eur Radiol. 2014; 24:2059–2068.33. Lee ES, Lee JY, Kim H, Choi Y, Park J, Han JK, et al. Pulsed high-intensity focused ultrasound enhances apoptosis of pancreatic cancer xenograft with gemcitabine. Ultrasound Med Biol. 2013; 39:1991–2000.34. Greis C. Technology overview: SonoVue (Bracco, Milan). Eur Radiol. 2004; 14:Suppl 8. P11–P15.35. He W, Wang W, Zhou P, Wang YX, Zhou P, Li RZ, et al. Enhanced ablation of high intensity focused ultrasound with microbubbles: an experimental study on rabbit hepatic VX2 tumors. Cardiovasc Intervent Radiol. 2011; 34:1050–1057.36. Chung DJ, Cho SH, Lee JM, Hahn ST. Effect of microbubble contrast agent during high intensity focused ultrasound ablation on rabbit liver in vivo. Eur J Radiol. 2012; 81:e519–e523.37. Poff JA, Allen CT, Traughber B, Colunga A, Xie J, Chen Z, et al. Pulsed high-intensity focused ultrasound enhances apoptosis and growth inhibition of squamous cell carcinoma xenografts with proteasome inhibitor bortezomib. Radiology. 2008; 248:485–491.38. Yu T, Wang G, Hu K, Ma P, Bai J, Wang Z. A microbubble agent improves the therapeutic efficiency of high intensity focused ultrasound: a rabbit kidney study. Urol Res. 2004; 32:14–19.39. Kaneko Y, Maruyama T, Takegami K, Watanabe T, Mitsui H, Hanajiri K, et al. Use of a microbubble agent to increase the effects of high intensity focused ultrasound on liver tissue. Eur Radiol. 2005; 15:1415–1420.40. Luo W, Zhou X, Ren X, Zheng M, Zhang J, He G. Enhancing effects of SonoVue, a microbubble sonographic contrast agent, on high-intensity focused ultrasound ablation in rabbit livers in vivo. J Ultrasound Med. 2007; 26:469–476.41. Luo W, Zhou X, Zhang J, Qian Y, Zheng M, Yu M, et al. Analysis of apoptosis and cell proliferation after high intensity-focused ultrasound ablation combined with microbubbles in rabbit livers. Eur J Gastroenterol Hepatol. 2007; 19:962–968.42. Vykhodtseva N, McDannold N, Martin H, Bronson RT, Hynynen K. Apoptosis in ultrasound-produced threshold lesions in the rabbit brain. Ultrasound Med Biol. 2001; 27:111–117.43. Kennedy JE, Ter Haar GR, Cranston D. High intensity focused ultrasound: surgery of the future. Br J Radiol. 2003; 76:590–599.44. Hilger I, Rapp A, Greulich KO, Kaiser WA. Assessment of DNA damage in target tumor cells after thermoablation in mice. Radiology. 2005; 237:500–506.45. Casey G, Cashman JP, Morrissey D, Whelan MC, Larkin JO, Soden DM, et al. Sonoporation mediated immunogene therapy of solid tumors. Ultrasound Med Biol. 2010; 36:430–440.46. Jiang L, Hu B, Guo Q, Chen L. Treatment of pancreatic cancer in a nude mouse model using high-intensity focused ultrasound. Exp Ther Med. 2013; 5:39–44.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Enhanced Chemotherapeutic Drug Delivery to Tumor Tissue by High Intensity Focused Ultrasound
- Current and Future Clinical Applications of High-Intensity Focused Ultrasound (HIFU) for Pancreatic Cancer
- Concurrent Chemotherapy and Pulsed High-Intensity Focused Ultrasound Therapy for the Treatment of Unresectable Pancreatic Cancer: Initial Experiences
- Magnetic Resonance-Guided Focused Ultrasound : Current Status and Future Perspectives in Thermal Ablation and Blood-Brain Barrier Opening
- High-intensity Focused Ultrasound Treatment (HIFU) for the Advanced Pancreatic Cancer