Korean J Radiol.
2016 Oct;17(5):715-724. 10.3348/kjr.2016.17.5.715.
Assessment of Blood-Brain Barrier Permeability by Dynamic Contrast-Enhanced MRI in Transient Middle Cerebral Artery Occlusion Model after Localized Brain Cooling in Rats
- Affiliations
-
- 1Department of Radiology, Hallym University Sacred Heart Hospital, Hallym University College of Medicine, Anyang 14068, Korea.
- 2Department of Radiology, Yonsei University College of Medicine, Seoul 03722, Korea. slee@yuhs.ac
- 3Department of Pathology, Hallym University Sacred Heart Hospital, Hallym University College of Medicine, Anyang 14068, Korea.
- 4Department of Neurology, Yonsei University College of Medicine, Seoul 03722, Korea.
- 5Department of Industrial Medicine, Hallym University Sacred Heart Hospital, Hallym University College of Medicine, Anyang 14068, Korea.
- 6Department of Radiology, Hallym University Kangdong Sacred Heart Hospital, Hallym University College of Medicine, Seoul 05355, Korea.
- 7Department of Radiology, Kangnam Sacred Heart Hospital, Hallym University College of Medicine, Seoul 07441, Korea.
- KMID: 2458063
- DOI: http://doi.org/10.3348/kjr.2016.17.5.715
Abstract
OBJECTIVE
The purpose of this study was to evaluate the effects of localized brain cooling on blood-brain barrier (BBB) permeability following transient middle cerebral artery occlusion (tMCAO) in rats, by using dynamic contrast-enhanced (DCE)-MRI.
MATERIALS AND METHODS
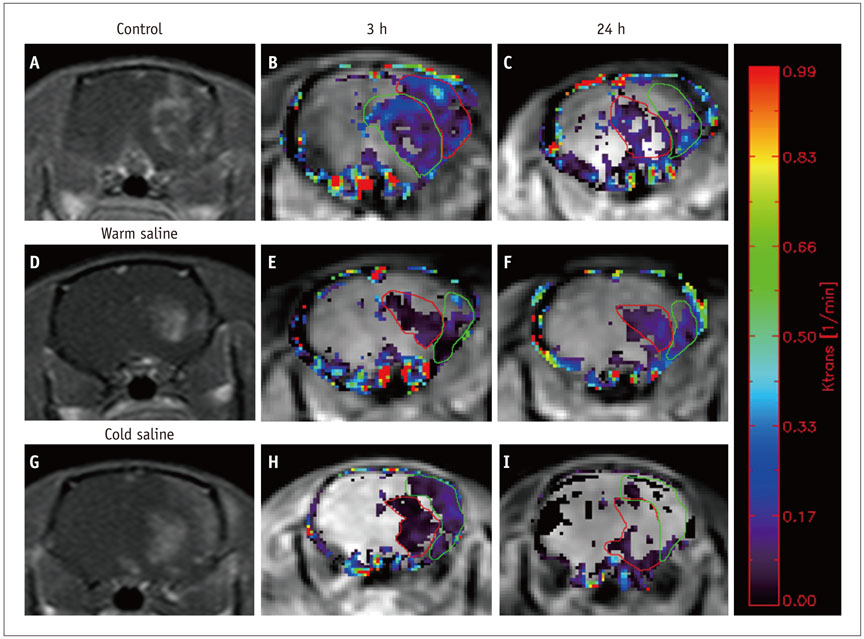
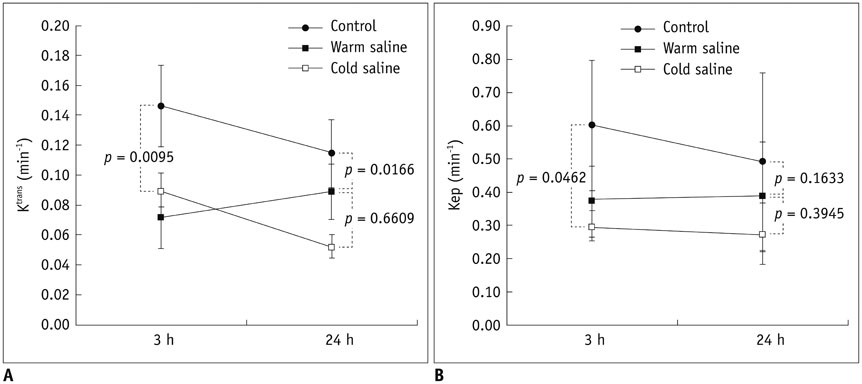
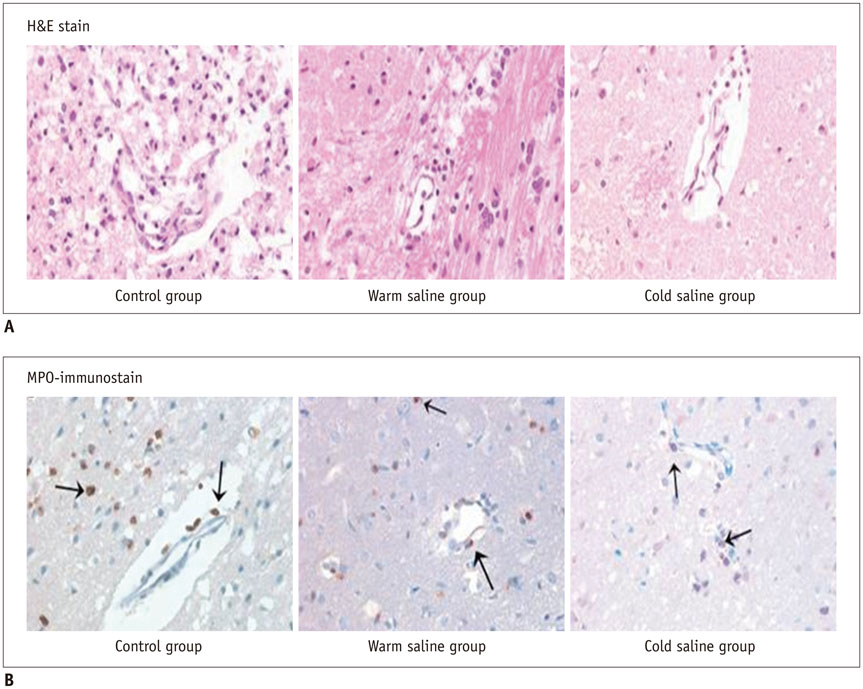
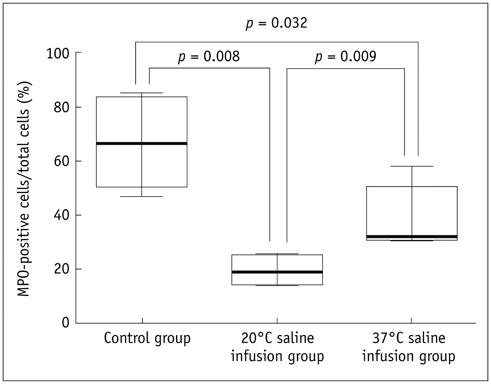
Thirty rats were divided into 3 groups of 10 rats each: control group, localized cold-saline (20℃) infusion group, and localized warm-saline (37℃) infusion group. The left middle cerebral artery (MCA) was occluded for 1 hour in anesthetized rats, followed by 3 hours of reperfusion. In the localized saline infusion group, 6 mL of cold or warm saline was infused through the hollow filament for 10 minutes after MCA occlusion. DCE-MRI investigations were performed after 3 hours and 24 hours of reperfusion. Pharmacokinetic parameters of the extended Tofts-Kety model were calculated for each DCE-MRI. In addition, rotarod testing was performed before tMCAO, and on days 1-9 after tMCAO. Myeloperoxidase (MPO) immunohisto-chemistry was performed to identify infiltrating neutrophils associated with the inflammatory response in the rat brain.
RESULTS
Permeability parameters showed no statistical significance between cold and warm saline infusion groups after 3-hour reperfusion 0.09 ± 0.01 min-1 vs. 0.07 ± 0.02 min-1, p = 0.661 for K(trans); 0.30 ± 0.05 min-1 vs. 0.37 ± 0.11 min-1, p = 0.394 for kep, respectively. Behavioral testing revealed no significant difference among the three groups. However, the percentage of MPO-positive cells in the cold-saline group was significantly lower than those in the control and warm-saline groups (p < 0.05).
CONCLUSION
Localized brain cooling (20℃) does not confer a benefit to inhibit the increase in BBB permeability that follows transient cerebral ischemia and reperfusion in an animal model, as compared with localized warm-saline (37℃) infusion group.
Keyword
MeSH Terms
-
Animals
Blood-Brain Barrier/*physiology
Brain/diagnostic imaging
Disease Models, Animal
Hypothermia, Induced/*methods
Infarction, Middle Cerebral Artery/diagnostic imaging/pathology/*physiopathology
Ischemic Attack, Transient/diagnostic imaging/physiopathology
Magnetic Resonance Imaging/methods
Male
Permeability
Rats, Sprague-Dawley
Reperfusion Injury/diagnostic imaging/physiopathology/prevention & control
Sodium Chloride
Sodium Chloride
Figure
Reference
-
1. Kataoka K, Yanase H. Mild hypothermia--a revived countermeasure against ischemic neuronal damages. Neurosci Res. 1998; 32:103–117.2. Chopp M, Knight R, Tidwell CD, Helpern JA, Brown E, Welch KM. The metabolic effects of mild hypothermia on global cerebral ischemia and recirculation in the cat: comparison to normothermia and hyperthermia. J Cereb Blood Flow Metab. 1989; 9:141–148.3. Colbourne F, Sutherland G, Corbett D. Postischemic hypothermia. A critical appraisal with implications for clinical treatment. Mol Neurobiol. 1997; 14:171–201.4. Barone FC, Feuerstein GZ, White RF. Brain cooling during transient focal ischemia provides complete neuroprotection. Neurosci Biobehav Rev. 1997; 21:31–44.5. Ginsberg MD, Sternau LL, Globus MY, Dietrich WD, Busto R. Therapeutic modulation of brain temperature: relevance to ischemic brain injury. Cerebrovasc Brain Metab Rev. 1992; 4:189–225.6. Schwab S. Therapy of severe ischemic stroke: breaking theconventional thinking. Cerebrovasc Dis. 2005; 20:Suppl 2. 169–178.7. Kallmünzer B, Kollmar R. Temperature management in stroke - an unsolved, but important topic. Cerebrovasc Dis. 2011; 31:532–543.8. Schwab S, Schwarz S, Spranger M, Keller E, Bertram M, Hacke W. Moderate hypothermia in the treatment of patients with severe middle cerebral artery infarction. Stroke. 1998; 29:2461–2466.9. Huang FP, Zhou LF, Yang GY. The effect of extending mild hypothermia on focal cerebral ischemia and reperfusion in the rat. Neurol Res. 1998; 20:57–62.10. Colbourne F, Sutherland GR, Auer RN. An automated system for regulating brain temperature in awake and freely moving rodents. J Neurosci Methods. 1996; 67:185–190.11. Kawai N, Okauchi M, Morisaki K, Nagao S. Effects of delayed intraischemic and postischemic hypothermia on a focal model of transient cerebral ischemia in rats. Stroke. 2000; 31:1982–1989. discussion 1989.12. Maier CM, Sun GH, Kunis D, Yenari MA, Steinberg GK. Delayed induction and long-term effects of mild hypothermia in a focal model of transient cerebral ischemia: neurological outcome and infarct size. J Neurosurg. 2001; 94:90–96.13. Xue D, Huang ZG, Smith KE, Buchan AM. Immediate or delayed mild hypothermia prevents focal cerebral infarction. Brain Res. 1992; 587:66–72.14. Yanamoto H, Hong SC, Soleau S, Kassell NF, Lee KS. Mild postischemic hypothermia limits cerebral injury following transient focal ischemia in rat neocortex. Brain Res. 1996; 718:207–211.15. Hewawasam P, Ding M, Chen N, King D, Knipe J, Pajor L, et al. Synthesis of water-soluble prodrugs of BMS-191011: a maxi-K channel opener targeted for post-stroke neuroprotection. Bioorg Med Chem Lett. 2003; 13:1695–1698.16. Ding Y, Li J, Luan X, Lai Q, McAllister JP 2nd, Phillis JW, et al. Local saline infusion into ischemic territory induces regional brain cooling and neuroprotection in rats with transient middle cerebral artery occlusion. Neurosurgery. 2004; 54:956–964. discussion 964-965.17. Luan X, Li J, McAllister JP 2nd, Diaz FG, Clark JC, Fessler RD, et al. Regional brain cooling induced by vascular saline infusion into ischemic territory reduces brain inflammation in stroke. Acta Neuropathol. 2004; 107:227–234.18. Ding Y, Li J, Rafols JA, Phillis JW, Diaz FG. Prereperfusion saline infusion into ischemic territory reduces inflammatory injury after transient middle cerebral artery occlusion in rats. Stroke. 2002; 33:2492–2498.19. Zhang RL, Chopp M, Zhang ZG, Jiang Q, Ewing JR. A rat model of focal embolic cerebral ischemia. Brain Res. 1997; 766:83–92.20. Busto R, Dietrich WD, Globus MY, Ginsberg MD. Postischemic moderate hypothermia inhibits CA1 hippocampal ischemic neuronal injury. Neurosci Lett. 1989; 101:299–304.21. Kassner A, Roberts TP, Moran B, Silver FL, Mikulis DJ. Recombinant tissue plasminogen activator increases blood-brain barrier disruption in acute ischemic stroke: an MR imaging permeability study. AJNR Am J Neuroradiol. 2009; 30:1864–1869.22. Larsson HB, Courivaud F, Rostrup E, Hansen AE. Measurement of brain perfusion, blood volume, and blood-brain barrier permeability, using dynamic contrast-enhanced T(1)-weighted MRI at 3 tesla. Magn Reson Med. 2009; 62:1270–1281.23. Choi HS, Ahn SS, Shin NY, Kim J, Kim JH, Lee JE, et al. Permeability parameters measured with dynamic contrast-enhanced MRI: correlation with the extravasation of evans blue in a rat model of transient cerebral ischemia. Korean J Radiol. 2015; 16:791–797.24. Durukan A, Marinkovic I, Strbian D, Pitkonen M, Pedrono E, Soinne L, et al. Post-ischemic blood-brain barrier leakage in rats: one-week follow-up by MRI. Brain Res. 2009; 1280:158–165.25. Ding G, Jiang Q, Li L, Zhang L, Gang Zhang Z, Ledbetter KA, et al. Detection of BBB disruption and hemorrhage by Gd-DTPA enhanced MRI after embolic stroke in rat. Brain Res. 2006; 1114:195–203.26. Jahng GH, Li KL, Ostergaard L, Calamante F. Perfusion magnetic resonance imaging: a comprehensive update on principles and techniques. Korean J Radiol. 2014; 15:554–577.27. Longa EZ, Weinstein PR, Carlson S, Cummins R. Reversible middle cerebral artery occlusion without craniectomy in rats. Stroke. 1989; 20:84–91.28. Kim SJ, Choi CG, Kim JK, Yun SC, Jahng GH, Jeong HK, et al. Effects of MR parameter changes on the quantification of diffusion anisotropy and apparent diffusion coefficient in diffusion tensor imaging: evaluation using a diffusional anisotropic phantom. Korean J Radiol. 2015; 16:297–303.29. Tofts PS, Kermode AG. Measurement of the blood-brain barrier permeability and leakage space using dynamic MR imaging. 1. Fundamental concepts. Magn Reson Med. 1991; 17:357–367.30. Tofts PS, Brix G, Buckley DL, Evelhoch JL, Henderson E, Knopp MV, et al. Estimating kinetic parameters from dynamic contrast-enhanced T(1)-weighted MRI of a diffusable tracer: standardized quantities and symbols. J Magn Reson Imaging. 1999; 10:223–232.31. Gupta YK, Sinha K, Chaudhary G. Transient focal ischemia induces motor deficit but does not impair the cognitive function in middle cerebral artery occlusion model of stroke in rats. J Neurol Sci. 2002; 203-204:267–271.32. Chu HX, Kim HA, Lee S, Moore JP, Chan CT, Vinh A, et al. Immune cell infiltration in malignant middle cerebral artery infarction: comparison with transient cerebral ischemia. J Cereb Blood Flow Metab. 2014; 34:450–459.33. Zhou W, Liesz A, Bauer H, Sommer C, Lahrmann B, Valous N, et al. Postischemic brain infiltration of leukocyte subpopulations differs among murine permanent and transient focal cerebral ischemia models. Brain Pathol. 2013; 23:34–44.34. Walter B, Bauer R, Kuhnen G, Fritz H, Zwiener U. Coupling of cerebral blood flow and oxygen metabolism in infant pigs during selective brain hypothermia. J Cereb Blood Flow Metab. 2000; 20:1215–1224.35. Choi JH, Marshall RS, Neimark MA, Konstas AA, Lin E, Chiang YT, et al. Selective brain cooling with endovascular intracarotid infusion of cold saline: a pilot feasibility study. AJNR Am J Neuroradiol. 2010; 31:928–934.36. Huang ZG, Xue D, Preston E, Karbalai H, Buchan AM. Biphasic opening of the blood-brain barrier following transient focal ischemia: effects of hypothermia. Can J Neurol Sci. 1999; 26:298–304.37. Nguyen GT, Coulthard A, Wong A, Sheikh N, Henderson R, O’Sullivan JD, et al. Measurement of blood-brain barrier permeability in acute ischemic stroke using standard first-pass perfusion CT data. Neuroimage Clin. 2013; 2:658–662.38. Na DG, Sohn CH, Kim EY. Imaging-based management of acute ischemic stroke patients: current neuroradiological perspectives. Korean J Radiol. 2015; 16:372–390.39. Ding Y, Young CN, Li J, Luan X, McAllister JP 2nd, Clark JD, et al. Reduced inflammatory mediator expression by prereperfusion infusion into ischemic territory in rats: a real-time polymerase chain reaction analysis. Neurosci Lett. 2003; 353:173–176.40. Schwartz AE, Stone JG, Pile-Spellman J, Finck AD, Sandhu AA, Mongero LB, et al. Selective cerebral hypothermia by means of transfemoral internal carotid artery catheterization. Radiology. 1996; 201:571–572.41. Zhao WH, Ji XM, Ling F, Ding YC, Xing CH, Wu H, et al. Local mild hypothermia induced by intra-arterial cold saline infusion prolongs the time window of onset of reperfusion injury after transient focal ischemia in rats. Neurol Res. 2009; 31:43–51.42. Okamoto K, Nagao K, Miki T, Nitobe E, Arima K, Hayashi N. New hypothermia method using blood cooling system: MONAN and KANEM method. In : Hayashi N, editor. Brain Hypothermia. Tokyo: Springer-Verlag Tokyo;2000. p. 203–209.43. Wei XE, Zhang YZ, Li YH, Li MH, Li WB. Dynamics of rabbit brain edema in focal lesion and perilesion area after traumatic brain injury: a MRI study. J Neurotrauma. 2012; 29:2413–2420.44. Beaumont A, Marmarou A, Hayasaki K, Barzo P, Fatouros P, Corwin F, et al. The permissive nature of blood brain barrier (BBB) opening in edema formation following traumatic brain injury. Acta Neurochir Suppl. 2000; 76:125–129.45. Bisdas S, Naegele T, Ritz R, Dimostheni A, Pfannenberg C, Reimold M, et al. Distinguishing recurrent high-grade gliomas from radiation injury: a pilot study using dynamic contrast-enhanced MR imaging. Acad Radiol. 2011; 18:575–583.46. Calamante F, Gadian DG, Connelly A. Delay and dispersion effects in dynamic susceptibility contrast MRI: simulations using singular value decomposition. Magn Reson Med. 2000; 44:466–473.47. Thacker NA, Scott ML, Jackson A. Can dynamic susceptibility contrast magnetic resonance imaging perfusion data be analyzed using a model based on directional flow? J Magn Reson Imaging. 2003; 17:241–255.48. Kassner A, Annesley DJ, Zhu XP, Li KL, Kamaly-Asl ID, Watson Y, et al. Abnormalities of the contrast re-circulation phase in cerebral tumors demonstrated using dynamic susceptibility contrast-enhanced imaging: a possible marker of vascular tortuosity. J Magn Reson Imaging. 2000; 11:103–113.49. Weisskoff RM, Zuo CS, Boxerman JL, Rosen BR. Microscopic susceptibility variation and transverse relaxation: theory and experiment. Magn Reson Med. 1994; 31:601–610.50. Leach MO, Brindle KM, Evelhoch JL, Griffiths JR, Horsman MR, Jackson A, et al. The assessment of antiangiogenic and antivascular therapies in early-stage clinical trials using magnetic resonance imaging: issues and recommendations. Br J Cancer. 2005; 92:1599–1610.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Permeability Parameters Measured with Dynamic Contrast-Enhanced MRI: Correlation with the Extravasation of Evans Blue in a Rat Model of Transient Cerebral Ischemia
- Effect of Selective Brain Cooling During the Cerebral Ischemia on the Post: Ischemic Brain Water Content in the Rabbit
- Animal Model of Cerebral Ischemia
- Transient Global Aphasia with Hemiparesis Following Cerebral Angiography : Relationship to Blood Brain Barrier Disruption
- Contrast Medium-Induced Transient Neurologic Deteriorations Following Cerebral angiography and Coil Embolization for Unruptured Aneurysm: Report of Two Cases