J Korean Neurosurg Soc.
2017 Sep;60(5):504-510. 10.3340/jkns.2017.0101.009.
Morphological and Hemodynamic Parameters for Middle Cerebral Artery Bifurcation Aneurysm Rupture Risk Assessment
- Affiliations
-
- 1Department of Neurosurgery, Xuanwu Hospital, Capital Medical University, Beijing, China. xwzhanghq@163.com
- 2Department of Neurosurgery, Zaozhuang Municipal Hospital, Zaozhuang, China.
- 3Department of Pharmacy, Zaozhuang Municipal Hospital, Zaozhuang, China.
- 4Department of Neurosurgery, The 3rd Hospital of Xiamen, Xiamen, China.
- KMID: 2457956
- DOI: http://doi.org/10.3340/jkns.2017.0101.009
Abstract
OBJECTIVE
To investigate the morphological and hemodynamic parameters associated with middle cerebral artery (MCA)bifurcation aneurysm rupture.
METHODS
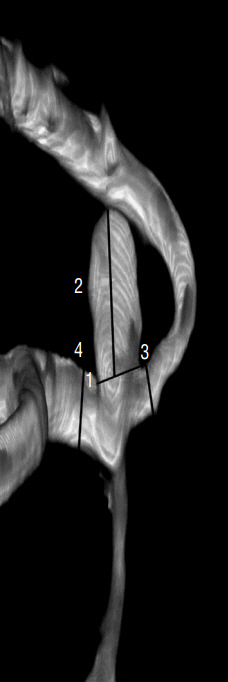
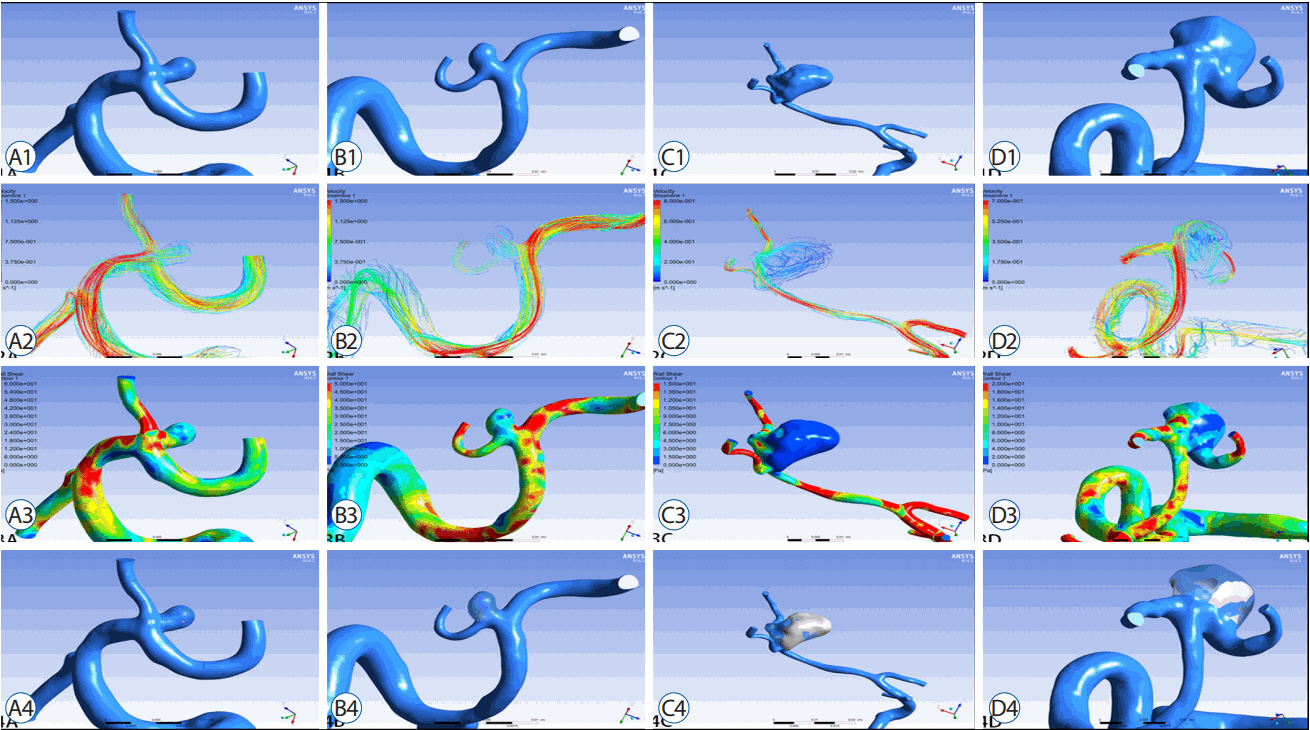
A retrospective study of 67 consecutive patients was carried out based on 3D digital subtraction angiography data. Morphological and hemodynamic parameters including aneurysm size parameters (dome width, height, and perpendicular height), longest dimension from the aneurysm neck to the dome tip, neck width, aneurysm area, aspect ratio, Longest dimension from the aneurysm neck to the dome tip (Dmax) to dome width, and height-width, Bottleneck factor, as well as wall shear stress (WSS), low WSS area (LSA), percentage of LSA (LSA%) and energy loss (EL) were estimated. Parameters between ruptured and un-ruptured groups were analyzed. Receiver operating characteristics were generated to check prediction performance of all significant variables.
RESULTS
Sixty-seven patients with MCA bifurcation aneurysm were included (31 unruptured, 36 ruptured). Dmax (p=0.008) was greater in ruptured group than that in un-ruptured group. D/W (p<0.001) and the percentage of the low WSS area (0.09±0.13 vs. 0.01±0.03, p<0.001) were also greater in the ruptured group. Moreover, the EL in ruptured group was higher than that in un-ruptured group (6.39±5.04 vs. 1.53±0.86, p<0.001). Multivariate regression analysis suggested D/W and EL were significant predictors of rupture of MCA bifurcation aneurysms. Correlation analyses revealed the D/W value was positively associated with the EL (R=0.442, p<0.01).
CONCLUSION
D/W and EL might be the most two favorable factors to predict rupture risk of MCA bifurcation aneurysms.
Keyword
MeSH Terms
Figure
Cited by 1 articles
-
Could A1 Aplasia or Hypoplasia Affect the Morphology and Rupture Risk of Anterior Communicating Artery Aneurysm?
Sung Chan Park, Na Young Jung, Eun Suk Park, Soon Chan Kwon
J Korean Neurosurg Soc. 2022;65(4):531-538. doi: 10.3340/jkns.2021.0283.
Reference
-
References
1. Connolly ES Jr, Rabinstein AA, Carhuapoma JR, Derdeyn CP, Dion J, Higashida RT, et al. Guidelines for the management of aneurysmal subarachnoid hemorrhage: a guideline for healthcare professionals from the American Heart Association/American Stroke Association. Stroke. 43:1711–1737. 2012.
Article2. Cross DT 3rd, Tirschwell DL, Clark MA, Tuden D, Derdeyn CP, Moran CJ, et al. Mortality rates after subarachnoid hemorrhage: variations according to hospital case volume in 18 states. J Neurosurg. 99:810–817. 2003.
Article3. Darsaut TE, Estrade L, Jamali S, Bojanowski MW, Chagnon M, Raymond J. Uncertainty and agreement in the management of unruptured intracranial aneurysms. J Neurosurg. 120:618–623. 2014.
Article4. Dashti R, Hernesniemi J, Niemelä M, Rinne J, Porras M, Lehecka M, et al. Microneurosurgical management of middle cerebral artery bifurcation aneurysms. Surg Neurol. 67:441–456. 2007.
Article5. Dhar S, Tremmel M, Mocco J, Kim M, Yamamoto J, Siddiqui AH, et al. Morphology parameters for intracranial aneurysm rupture risk assessment. Neurosurgery. 63:185–196. discussion 196–197. 2008.
Article6. Duan G, Lv N, Yin J, Xu J, Hong B, Xu Y, et al. Morphological and hemodynamic analysis of posterior communicating artery aneurysms prone to rupture: a matched case-control study. J Neurointerv Surg. 8:47–51. 2016.
Article7. Elsharkawy A, Lehečka M, Niemelä M, Kivelev J, Billon-Grand R, Lehto H, et al. Anatomic risk factors for middle cerebral artery aneurysm rupture: computed tomography angiography study of 1009 consecutive patients. Neurosurgery. 73:825–837. discussion 836–837. 2013.8. Farnoush A, Avolio A, Qian Y. A growth model of saccular aneurysms based on hemodynamic and morphologic discriminant parameters for risk of rupture. J Clin Neurosci. 21:1514–1519. 2014.
Article9. Fan J, Wang Y, Liu J, Jing L, Wang C, Li C, et al. Morphological-hemodynamic characteristics of intracranial bifurcation mirror aneurysms. World Neurosurg. 84:114–120.e2. 2015.
Article10. Hu P, Qian Y, Lee CJ, Zhang HQ, Ling F. The energy loss may predict rupture risks of anterior communicating aneurysms: a preliminary result. Int J Clin Exp Med. 8:4128–4133. 2015.11. Jing L, Zhong J, Liu J, Yang X, Paliwal N, Meng H, et al. Hemodynamic effect of flow diverter and coils in treatment of large and giant intracranial aneurysms. World Neurosurg. 89:199–207. 2016.
Article12. Lall RR, Eddleman CS, Bendok BR, Batjer HH. Unruptured intracranial aneurysms and the assessment of rupture risk based on anatomical and morphological factors: sifting through the sands of data. Neurosurg Focus. 26:E2. 2009.
Article13. Lauric A, Baharoglu MI, Malek AM. Ruptured status discrimination performance of aspect ratio, height/width, and bottleneck factor is highly dependent on aneurysm sizing methodology. Neurosurgery. 71:38–45. 2012.
Article14. Long Y, Zhong J, Yu H, Yan H, Zhuo Z, Meng Q, et al. A scaling aneurysm model-based approach to assessing the role of flow pattern and energy loss in aneurysm rupture prediction. J Transl Med. 13:311. 2015.
Article15. Lv N, Feng Z, Wang C, Cao W, Fang Y, Karmonik C, et al. Morphological risk factors for rupture of small (<7 mm) posterior communicating artery aneurysms. World Neurosurg. 87:311–315. 2016.
Article16. Lv N, Wang C, Karmonik C, Fang Y, Xu J, Yu Y, et al. Morphological and hemodynamic discriminators for rupture status in posterior communicating artery aneurysms. PLoS One. 11:e0149906. 2016.
Article17. Lv N, Yu Y, Xu J, Karmonik C, Liu J, Huang Q. Hemodynamic and morphological characteristics of unruptured posterior communicating artery aneurysms with oculomotor nerve palsy. J Neurosurg. 125:264–268. 2016.
Article18. Malek AM, Alper SL, Izumo S. Hemodynamic shear stress and its role in atherosclerosis. JAMA. 282:2035–2042. 1999.
Article19. Maiti TK, Bir SC, Patra DP, Cuellar H, Nanda A. 158 morphological parameters for anterior communicating artery aneurysm rupture risk assessment. Neurosurgery. 63(Suppl 1):163–164. 2016.
Article20. Meng H, Tutino VM, Xiang J, Siddiqui A. High WSS or low WSS? Complex interactions of hemodynamics with intracranial aneurysm initiation, growth, and rupture: toward a unifying hypothesis. AJNR Am J Neuroradiol. 35:1254–1262. 2014.
Article21. Miura Y, Ishida F, Umeda Y, Tanemura H, Suzuki H, Matsushima S, et al. Low wall shear stress is independently associated with the rupture status of middle cerebral artery aneurysms. Stroke. 44:519–521. 2013.
Article22. Pereira VM, Brina O, Gonzalez AM, Narata AP, Ouared R, Karl-Olof L. Biology and hemodynamics of aneurismal vasculopathies. Eur J Radiol. 82:1606–1617. 2013.
Article23. Qian Y, Takao H, Umezu M, Murayama Y. Risk analysis of unruptured aneurysms using computational fluid dynamics technology: preliminary results. AJNR Am J Neuroradiol. 32:1948–1955. 2011.
Article24. Shojima M, Oshima M, Takagi K, Torii R, Hayakawa M, Katada K, et al. Magnitude and role of wall shear stress on cerebral aneurysm: computational fluid dynamic study of 20 middle cerebral artery aneurysms. Stroke. 35:2500–2505. 2004.
Article25. Sugiyama S, Meng H, Funamoto K, Inoue T, Fujimura M, Nakayama T, et al. Hemodynamic analysis of growing intracranial aneurysms arising from a posterior inferior cerebellar artery. World Neurosurg. 78:462–468. 2012.
Article26. Takao H, Murayama Y, Otsuka S, Qian Y, Mohamed A, Masuda S, et al. Hemodynamic differences between unruptured and ruptured intracranial aneurysms during observation. Stroke. 43:1436–1439. 2012.
Article27. Taylor CA, Hughes TJ, Zarins CK. Finite element modeling of three-dimensional pulsatile flow in the abdominal aorta: relevance to atherosclerosis. Ann Biomed Eng. 26:975–987. 1998.
Article28. Ujiie H, Tachibana H, Hiramatsu O, Hazel AL, Matsumoto T, Ogasawara Y, et al. Effects of size and shape (aspect ratio) on the hemodynamics of saccular aneurysms: a possible index for surgical treatment of intracranial aneurysms. Neurosurgery. 45:119–129. discussion 129–130. 1999.
Article29. Ujiie H, Tamano Y, Sasaki K, Hori T. Is the aspect ratio a reliable index for predicting the rupture of a saccular aneurysm? Neurosurgery. 48:495–502. discussion 502–503. 2001.
Article30. Xiang J, Natarajan SK, Tremmel M, Ma D, Mocco J, Hopkins LN, et al. Hemodynamic-morphologic discriminants for intracranial aneurysm rupture. Stroke. 42:144–152. 2011.
Article31. Xiang J, Yu J, Choi H, Dolan Fox JM, Snyder KV, Levy EI, et al. Rupture Resemblance Score (RRS): toward risk stratification of unruptured intracranial aneurysms using hemodynamic-morphological discriminants. J Neurointerv Surg. 7:490–495. 2015.
Article32. Xiang J, Yu J, Snyder KV, Levy EI, Siddiqui AH, Meng H. Hemodynamic-morphological discriminant models for intracranial aneurysm rupture remain stable with increasing sample size. J Neurointerv Surg. 8:104–110. 2016.
Article33. Zhang Y, Jing L, Liu J, Li C, Fan J, Wang S, et al. Clinical, morphological, and hemodynamic independent characteristic factors for rupture of posterior communicating artery aneurysms. J Neurointerv Surg. 8:808–812. 2016.
Article34. Zhang Y, Mu S, Chen J, Wang S, Li H, Yu H, et al. Hemodynamic analysis of intracranial aneurysms with daughter blebs. Eur Neurol. 66:359–367. 2011.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Angiographic Analysis of Middle Cerebral Artery Bifurcation Aneurysm
- Subarachnoid Hemorrhage Due to a Ruptured Middle Cerebral Artery Bifurcation Aneurysm Superimposed by an Idiopathic Intracerebral Hematoma
- Traumatic Rupture of Middle Cerebral Artery Aneurysm
- De novo Formation of Familial Cerebral Aneurysms
- Middle Cerebral Artery Variations Associated with Intracranial Aneurysmal Rupture