Ann Lab Med.
2020 Jan;40(1):48-56. 10.3343/alm.2020.40.1.48.
Human B1 Cells are the Main Blood Group A-Specific B Cells That Have a Moderate Correlation With Anti-A Antibody Titer
- Affiliations
-
- 1Department of Preventive Medicine, Yanbian University College of Medicine, Yanji, Jilin, People's Republic of China. yrge@ybu.edu.cn
- 2Biomedical Research Institute, Seoul National University Hospital, Seoul, Korea.
- 3Transplantation Center, Seoul National University Hospital, Seoul, Korea. jcyjs@snu.ac.kr
- 4Department of Surgery, Seoul National University Hospital, Seoul, Korea.
- KMID: 2457493
- DOI: http://doi.org/10.3343/alm.2020.40.1.48
Abstract
- BACKGROUND
Anti-carbohydrate antibody responses, including those of anti-blood group ABO antibodies, are yet to be thoroughly studied in humans. Because anti-ABO antibody-mediated rejection is a key hurdle in ABO-incompatible transplantation, it is important to understand the cellular mechanism of anti-ABO responses. We aimed to identify the main human B cell subsets that produce anti-ABO antibodies by analyzing the correlation between B cell subsets and anti-ABO antibody titers.
METHODS
Blood group A-binding B cells were analyzed in peritoneal fluid and peripheral blood samples from 43 patients undergoing peritoneal dialysis and 18 healthy volunteers with blood group B or O. The correlation between each blood group A-specific B cell subset and anti-A antibody titer was then analyzed using Pearson's correlation analysis.
RESULTS
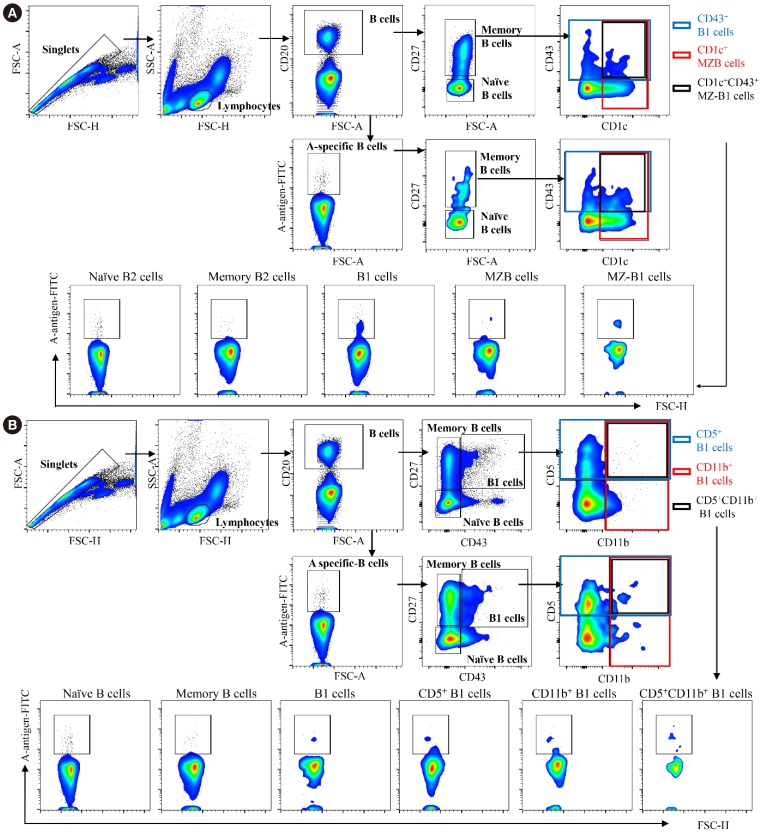
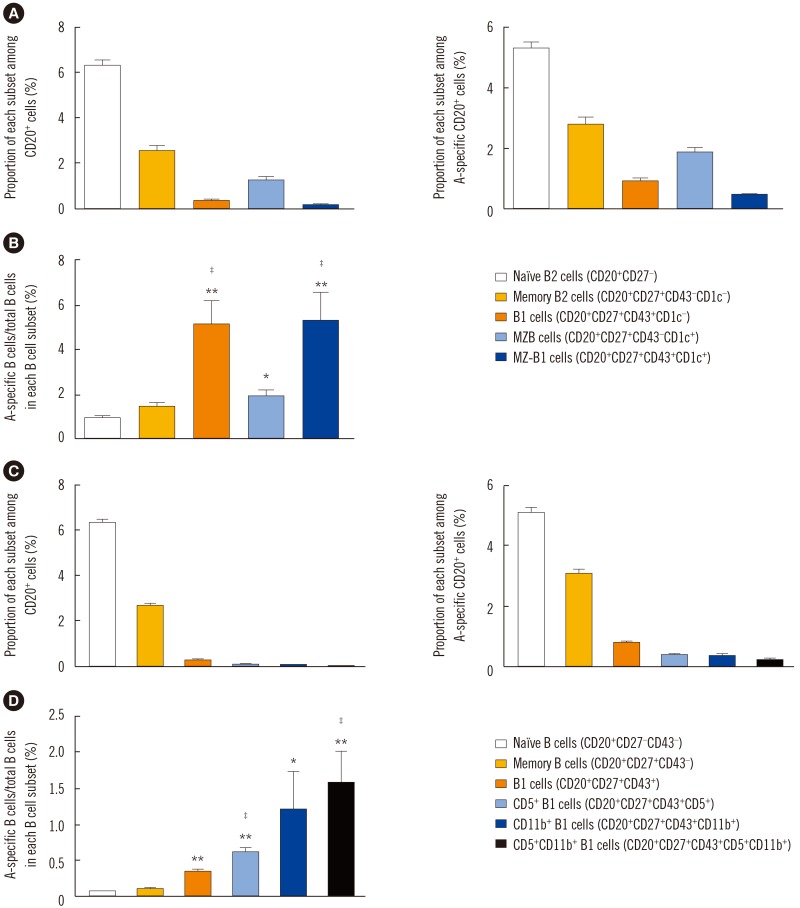
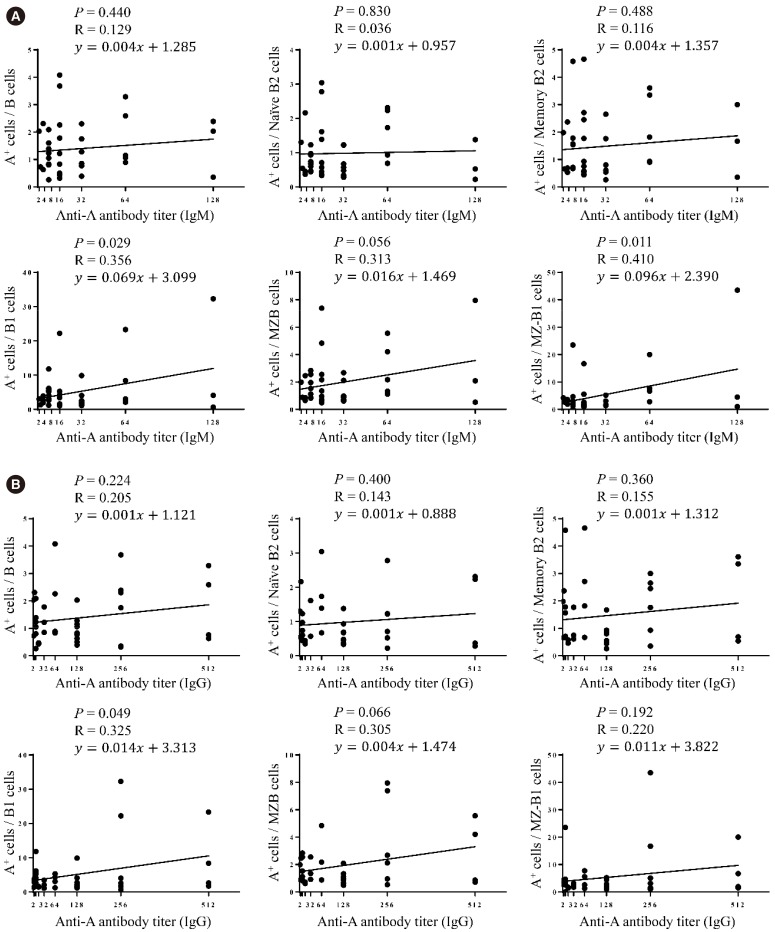
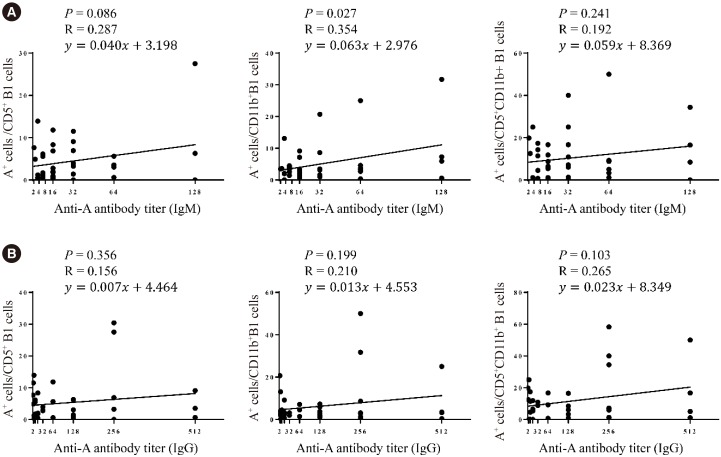
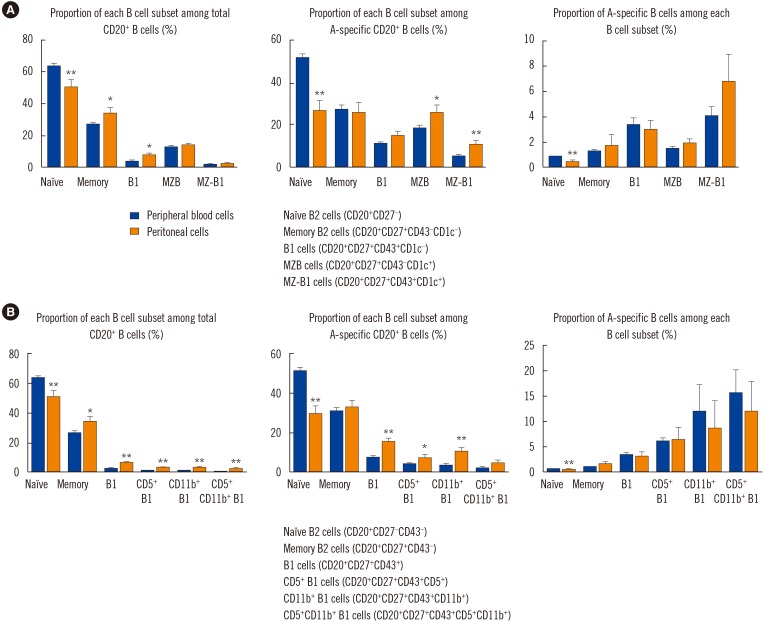
Blood group A-binding B cells were enriched in CD27âºCD43âºCD1c− B1, CD5⺠B1, CD11b⺠B1, and CD27âºCD43âºCD1c+ marginal zone-B1 cells in peripheral blood. Blood group A-specific B1 cells (P=0.029 and R=0.356 for IgM; P=0.049 and R=0.325 for IgG) and marginal zone-B1 cells (P=0.011 and R=0.410 for IgM) were positively correlated with anti-A antibody titer. Further analysis of peritoneal B cells confirmed B1 cell enrichment in the peritoneal cavity but showed no difference in blood group A-specific B1 cell enrichment between the peritoneal cavity and peripheral blood.
CONCLUSIONS
Human B1 cells are the key blood group A-specific B cells that have a moderate correlation with anti-A antibody titer and therefore constitute a potential therapeutic target for successful ABO-incompatible transplantation.
Keyword
MeSH Terms
Figure
Reference
-
1. Morath C, Zeier M, Döhler B, Opelz G, Süsal C. ABO-incompatible kidney transplantation. Front Immunol. 2017; 8:234. PMID: 28321223.2. Okumi M, Toki D, Nozaki T, Shimizu T, Shirakawa H, Omoto K, et al. ABO-incompatible living kidney transplants: evolution of outcomes and immunosuppressive management. Am J Transplant. 2016; 16:886–896. PMID: 26555133.3. Segev DL, Gentry SE, Melancon JK, Montgomery RA. Characterization of waiting times in a simulation of kidney paired donation. Am J Transplant. 2005; 5:2448–2455. PMID: 16162194.4. Mond JJ, Lees A, Snapper CM. T cell-independent antigens type 2. Annu Rev Immunol. 1995; 13:655–692. PMID: 7612238.5. Tazawa H, Irei T, Tanaka Y, Igarashi Y, Tashiro H, Ohdan H. Blockade of invariant TCR-CD1d interaction specifically inhibits antibody production against blood group A carbohydrates. Blood. 2013; 122:2582–2590. PMID: 23943651.6. Irei T, Ohdan H, Zhou W, Ishiyama K, Tanaka Y, Ide K, et al. The persistent elimination of B cells responding to blood group A carbohydrates by synthetic group A carbohydrates and B-1 cell differentiation blockade: novel concept in preventing antibody-mediated rejection in ABO-incompatible transplantation. Blood. 2007; 110:4567–4575. PMID: 17766679.7. Zhou W, Ohdan H, Tanaka Y, Hara H, Tokita D, Onoe T, et al. NOD/SCID mice engrafted with human peripheral blood lymphocytes can be a model for investigating B cells responding to blood group A carbohydrate determinant. Transpl Immunol. 2003; 12:9–18. PMID: 14551028.8. Hardy RR. B-1 B cell development. J Immunol. 2006; 177:2749–2754. PMID: 16920907.9. Griffin DO, Holodick NE, Rothstein TL. Human B1 cells in umbilical cord and adult peripheral blood express the novel phenotype CD20+ CD27+ CD43+ CD70−. J Exp Med. 2011; 208:67–80. PMID: 21220451.10. Griffin DO, Rothstein TL. A small CD11b(+) human B1 cell subpopulation stimulates T cells and is expanded in lupus. J Exp Med. 2011; 208:2591–2598. PMID: 22110167.11. Verbinnen B, Covens K, Moens L, Meyts I, Bossuyt X. Human CD20+ CD43+CD27+CD5− B cells generate antibodies to capsular polysaccharides of Streptococcus pneumoniae. J Allergy Clin Immunol. 2012; 130:272–275. PMID: 22664161.12. Xu H, Sharma A, Lei Y, Okabe J, Wan H, Chong AS, et al. Development and characterization of anti-Gal B cell receptor transgenic Gal-/- mice. Transplantation. 2002; 73:1549–1557. PMID: 12042639.13. Weill JC, Weller S, Reynaud CA. Human marginal zone B cells. Annu Rev Immunol. 2009; 27:267–285. PMID: 19302041.14. Weller S, Braun MC, Tan BK, Rosenwald A, Cordier C, Conley ME, et al. Human blood IgM ‘memory’ B cells are circulating splenic marginal zone B cells harboring a prediversified immunoglobulin repertoire. Blood. 2004; 104:3647–3654. PMID: 15191950.15. Wolfram W, Sauerwein KM, Binder CJ, Eibl-Musil N, Wolf HM, Fischer MB. Pneumococcal polysaccharide vaccination elicits IgG anti-A/B blood group antibodies in healthy individuals and patients with type I diabetes mellitus. Front Immunol. 2016; 7:493. PMID: 27895641.16. Akoglu H. User's guide to correlation coefficients. Turk J Emerg Med. 2018; 18:91–93. PMID: 30191186.17. Koo TY, Yang J. Current progress in ABO-incompatible kidney transplantation. Kidney Res Clin Pract. 2015; 34:170–179. PMID: 26484043.18. Aziz M, Holodick NE, Rothstein TL, Wang P. The role of B-1 cells in inflammation. Immunol Res. 2015; 63:153–166. PMID: 26427372.19. Quách TD, Rodríguez-Zhurbenko N, Hopkins TJ, Guo X, Hernández AM, Li W, et al. Distinctions among circulating antibody-secreting cell populations, including B-1 cells, in human adult peripheral blood. J Immunol. 2016; 196:1060–1069. PMID: 26740107.20. Lee JG, Jang JY, Fang T, Xu Y, Yan JJ, Ryu JH, et al. Identification of human B-1 helper T cells with a Th1-like memory phenotype and high integrin CD49d expression. Front Immunol. 2018; 9:1617. PMID: 30061889.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Eukaryotic Expression of Human Cytomegalovirus ( HCMV ) Glycoprotein H ( gH )
- Eukaryotic Kxpression of the Major Antigenic Determinants Evoking Neutralizing Antibodies in Human Cytomegalovirus ( HCMV ) Isolated in Korea
- Association between interstitial cells of Cajal and anti-vinculin antibody in human stomach
- A Case of Anti-Yka Antibody as an High-Titer, Low-Avidity Antibody: The First Case in Korea
- Comparison of ABO Antibody Titers on the Basis of the Antibody Detection Method Used