Neonatal Med.
2019 Aug;26(3):121-127. 10.5385/nm.2019.26.3.121.
Clinical Application of Near-Infrared Spectroscopy in Neonates
- Affiliations
-
- 1Department of Pediatrics, Inje University Busan Paik Hospital, Inje University College of Medicine, Busan, Korea. iamgawon@paik.ac.kr
- KMID: 2457179
- DOI: http://doi.org/10.5385/nm.2019.26.3.121
Abstract
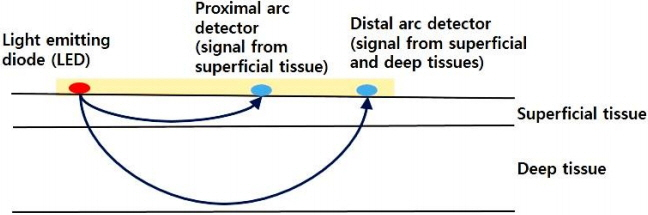
- The incidence of cerebral palsy has not decreased despite advances in neonatal care. Preterm infants are at a high risk of cerebral palsy. Moreover, preterm infants might experience permanent neurological sequelae due to injury in the preterm brain. Although the etiology of preterm brain injury is not fully understood, preterm brain injury is strongly associated with abnormal cerebral perfusion and oxygenation. Monitoring systemic blood pressure or arterial oxygen saturation using pulse oximetry is not enough to guarantee proper cerebral perfusion or oxygenation. Early detection of improper cerebral perfusion can prevent irreversible cerebral damage. To decrease brain injury through the early detection of under-perfusion and deoxygenation, other diagnostic modalities are needed. Near-infrared spectroscopy can continuously and noninvasively monitor regional oxygen saturation (rSOâ‚‚), which reflects the perfusion and oxygenation status of tissues at bedside. Near-infrared spectroscopy represents a balance between tissue oxygen supply and demand. Cerebral rSOâ‚‚ monitoring has been used most frequently in neonatal cardiac surgery to monitor cerebral oxygenation and prevent hypoxic damage or shock. Recently, cerebral, renal, or splanchnic rSOâ‚‚ in neonates is frequently monitored. The progression of a disease, brain injury, and death can be prevented by detecting changes in rSOâ‚‚ values using near-infrared spectroscopy. In this article, the basic principles, usefulness, and applications of near-infrared spectroscopy in neonates are discussed.
Keyword
MeSH Terms
Figure
Reference
-
1. Novak I, Morgan C, Adde L, Blackman J, Boyd RN, Brunstrom-Hernandez J, et al. Early, accurate diagnosis and early intervention in cerebral palsy: advances in diagnosis and treatment. JAMA Pediatr. 2017; 171:897–907.2. Babcock MA, Kostova FV, Ferriero DM, Johnston MV, Brunstrom JE, Hagberg H, et al. Injury to the preterm brain and cerebral palsy: clinical aspects, molecular mechanisms, unanswered questions, and future research directions. J Child Neurol. 2009; 24:1064–84.3. Van Bel F, Van de Bor M, Stijnen T, Baan J, Ruys JH. Aetiological role of cerebral blood-flow alterations in development and extension of peri-intraventricular haemorrhage. Dev Med Child Neurol. 1987; 29:601–14.4. Verhagen EA, Van Braeckel KN, van der Veere CN, Groen H, Dijk PH, Hulzebos CV, et al. Cerebral oxygenation is associated with neurodevelopmental outcome of preterm children at age 2 to 3 years. Dev Med Child Neurol. 2015; 57:449–55.5. Børch K, Lou HC, Greisen G. Cerebral white matter blood flow and arterial blood pressure in preterm infants. Acta Paediatr. 2010; 99:1489–92.6. Wong FY, Silas R, Hew S, Samarasinghe T, Walker AM. Cerebral oxygenation is highly sensitive to blood pressure variability in sick preterm infants. PLoS One. 2012; 7:e43165.7. Marin T, Moore J. Understanding near-infrared spectroscopy. Adv Neonatal Care. 2011; 11:382–8.8. Watzman HM, Kurth CD, Montenegro LM, Rome J, Steven JM, Nicolson SC. Arterial and venous contributions to near-infrared cerebral oximetry. Anesthesiology. 2000; 93:947–53.9. Sood BG, McLaughlin K, Cortez J. Near-infrared spectroscopy: applications in neonates. Semin Fetal Neonatal Med. 2015; 20:164–72.10. Grometto A, Pizzo B, Strozzi MC, Gazzolo F, Gazzolo D. Cerebral NIRS patterns in late preterm and very preterm infants becoming late preterm. J Matern Fetal Neonatal Med. 2019; 32:1124–9.11. Garvey AA, Dempsey EM. Applications of near infrared spectroscopy in the neonate. Curr Opin Pediatr. 2018; 30:209–15.12. McCormick PW, Stewart M, Goetting MG, Dujovny M, Lewis G, Ausman JI. Noninvasive cerebral optical spectroscopy for monitoring cerebral oxygen delivery and hemodynamics. Crit Care Med. 1991; 19:89–97.13. Lemmers PM, Toet M, van Schelven LJ, van Bel F. Cerebral oxygenation and cerebral oxygen extraction in the preterm infant: the impact of respiratory distress syndrome. Exp Brain Res. 2006; 173:458–67.14. Petrova A, Mehta R. Near-infrared spectroscopy in the detection of regional tissue oxygenation during hypoxic events in preterm infants undergoing critical care. Pediatr Crit Care Med. 2006; 7:449–54.15. McNeill S, Gatenby JC, McElroy S, Engelhardt B. Normal cerebral, renal and abdominal regional oxygen saturations using nearinfrared spectroscopy in preterm infants. J Perinatol. 2011; 31:51–7.16. Sorensen LC, Leung TS, Greisen G. Comparison of cerebral oxygen saturation in premature infants by near-infrared spatially resolved spectroscopy: observations on probe-dependent bias. J Biomed Opt. 2008; 13:064013.17. Kishi K, Kawaguchi M, Yoshitani K, Nagahata T, Furuya H. Influence of patient variables and sensor location on regional cerebral oxygen saturation measured by INVOS 4100 near-infrared spectrophotometers. J Neurosurg Anesthesiol. 2003; 15:302–6.18. Schwaberger B, Pichler G, Binder-Heschl C, Baik N, Avian A, Urlesberger B. Transitional changes in cerebral blood volume at birth. Neonatology. 2015; 108:253–8.19. Kenosi M, O'Toole JM, Livingston V, Hawkes GA, Boylan GB, O'Halloran KD, et al. Effects of fractional inspired oxygen on cerebral oxygenation in preterm infants following delivery. J Pediatr. 2015; 167:1007–12.20. van Vonderen JJ, Roest AA, Siew ML, Walther FJ, Hooper SB, te Pas AB. Measuring physiological changes during the transition to life after birth. Neonatology. 2014; 105:230–42.21. Pichler G, Binder C, Avian A, Beckenbach E, Schmolzer GM, Urlesberger B. Reference ranges for regional cerebral tissue oxygen saturation and fractional oxygen extraction in neonates during immediate transition after birth. J Pediatr. 2013; 163:1558–63.22. Sorensen LC, Greisen G. The brains of very preterm newborns in clinically stable condition may be hyperoxygenated. Pediatrics. 2009; 124:e958. –63.23. Toet MC, Lemmers PM. Brain monitoring in neonates. Early Hum Dev. 2009; 85:77–84.24. Toet MC, Lemmers PM, van Schelven LJ, van Bel F. Cerebral oxygenation and electrical activity after birth asphyxia: their relation to outcome. Pediatrics. 2006; 117:333–9.25. Wintermark P, Hansen A, Warfield SK, Dukhovny D, Soul JS. Near-infrared spectroscopy versus magnetic resonance imaging to study brain perfusion in newborns with hypoxic-ischemic encephalopathy treated with hypothermia. Neuroimage. 2014; 85 Pt 1:287–93.26. Alderliesten T, Dix L, Baerts W, Caicedo A, van Huffel S, Naulaers G, et al. Reference values of regional cerebral oxygen saturation during the first 3 days of life in preterm neonates. Pediatr Res. 2016; 79:55–64.27. Pellicer A, Greisen G, Benders M, Claris O, Dempsey E, Fumagalli M, et al. The SafeBoosC phase II randomised clinical trial: a treatment guideline for targeted near-infrared-derived cerebral tissue oxygenation versus standard treatment in extremely preterm infants. Neonatology. 2013; 104:171–8.28. Tina LG, Frigiola A, Abella R, Artale B, Puleo G, D'Angelo S, et al. Near infrared spectroscopy in healthy preterm and term newborns: correlation with gestational age and standard monitoring parameters. Curr Neurovasc Res. 2009; 6:148–54.29. Wong FY, Leung TS, Austin T, Wilkinson M, Meek JH, Wyatt JS, et al. Impaired autoregulation in preterm infants identified by using spatially resolved spectroscopy. Pediatrics. 2008; 121:e604. –11.30. Riera J, Cabanas F, Serrano JJ, Madero R, Pellicer A. New developments in cerebral blood flow autoregulation analysis in preterm infants: a mechanistic approach. Pediatr Res. 2016; 79:460–5.31. Lemmers PM, Benders MJ, D'Ascenzo R, Zethof J, Alderliesten T, Kersbergen KJ, et al. Patent ductus arteriosus and brain volume. Pediatrics. 2016; 137:e20153090.32. Lemmers PM, Toet MC, van Bel F. Impact of patent ductus arteriosus and subsequent therapy with indomethacin on cerebral oxygenation in preterm infants. Pediatrics. 2008; 121:142–7.33. Schwarz CE, Preusche A, Wolf M, Poets CF, Franz AR. Prospective observational study on assessing the hemodynamic relevance of patent ductus arteriosus with frequency domain near-infrared spectroscopy. BMC Pediatr. 2018; 18:66.34. Patel J, Roberts I, Azzopardi D, Hamilton P, Edwards AD. Randomized double-blind controlled trial comparing the effects of ibuprofen with indomethacin on cerebral hemodynamics in preterm infants with patent ductus arteriosus. Pediatr Res. 2000; 47:36–42.35. Underwood MA, Milstein JM, Sherman MP. Near-infrared spectroscopy as a screening tool for patent ductus arteriosus in extremely low birth weight infants. Neonatology. 2007; 91:134–9.36. Huning BM, Asfour B, Konig S, Hess N, Roll C. Cerebral blood volume changes during closure by surgery of patent ductus arteriosus. Arch Dis Child Fetal Neonatal Ed. 2008; 93:F261–4.37. Lemmers PM, Molenschot MC, Evens J, Toet MC, van Bel F. Is cerebral oxygen supply compromised in preterm infants undergoing surgical closure for patent ductus arteriosus? Arch Dis Child Fetal Neonatal Ed. 2010; 95:F429–34.38. Vanderhaegen J, De Smet D, Meyns B, Van De Velde M, Van Huffel S, Naulaers G. Surgical closure of the patent ductus arteriosus and its effect on the cerebral tissue oxygenation. Acta Paediatr. 2008; 97:1640–4.39. Uebing A, Furck AK, Hansen JH, Nufer E, Scheewe J, Dutschke P, et al. Perioperative cerebral and somatic oxygenation in neonates with hypoplastic left heart syndrome or transposition of the great arteries. J Thorac Cardiovasc Surg. 2011; 142:523–30.40. Hansen JH, Schlangen J, Voges I, Jung O, Wegmann A, Scheewe J, et al. Impact of afterload reduction strategies on regional tissue oxygenation after the Norwood procedure for hypoplastic left heart syndrome. Eur J Cardiothorac Surg. 2014; 45:e13. –9.41. Phelps HM, Mahle WT, Kim D, Simsic JM, Kirshbom PM, Kanter KR, et al. Postoperative cerebral oxygenation in hypoplastic left heart syndrome after the Norwood procedure. Ann Thorac Surg. 2009; 87:1490–4.42. Patel AK, Lazar DA, Burrin DG, Smith EO, Magliaro TJ, Stark AR, et al. Abdominal near-infrared spectroscopy measurements are lower in preterm infants at risk for necrotizing enterocolitis. Pediatr Crit Care Med. 2014; 15:735–41.43. Schat TE, Schurink M, van der Laan ME, Hulscher JB, Hulzebos CV, Bos AF, et al. Near-infrared spectroscopy to predict the course of necrotizing enterocolitis. PLoS One. 2016; 11:e0154710.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Clinical Application of Near Infrared Spectroscopy
- Application of near-infrared spectroscopy in clinical neurology
- Two-channel Near-infrared Spectroscopic Analysis of Association of Paranoia Symptoms with Prefrontal Activation
- Calculi in Hydrocele: Incidence and Results of Infrared Spectroscopy Analysis
- Surgical Treatment of an Innominate Artery Aneurysm Using Near-Infrared Spectroscopy for Cerebral Monitoring: A Case Report