Korean Circ J.
2019 Aug;49(8):742-752. 10.4070/kcj.2018.0361.
The Burden and Risk Factors for Infection of Transvenous Cardiovascular Implantable Electronic Device: a Nationwide Cohort Study
- Affiliations
-
- 1Department of Cardiology, CHA Bundang Medical Center, CHA University, Seongnam, Korea.
- 2Medtronic Korea, Ltd., Seoul, Korea. hyung.deuk.park@medtronic.com
- 3Division of Cardiology, Department of Internal Medicine, Severance Cardiovascular Hospital, Yonsei University College of Medicine, Seoul, Korea. cby6908@yuhs.ac
- 4Graduate School for Medical Device Management and Research, Samsung Advanced Institute for Health Science & Technology (SAIHST), Sungkyunkwan University, Seoul, Korea.
- KMID: 2456869
- DOI: http://doi.org/10.4070/kcj.2018.0361
Abstract
- BACKGROUND AND OBJECTIVES
There are limited published data on the incidence and cost associated with cardiac implantable electrical device (CIED) infection for Asian patients. We analyzed the infection burden associated with the implantation of CIEDs in Korea.
METHODS
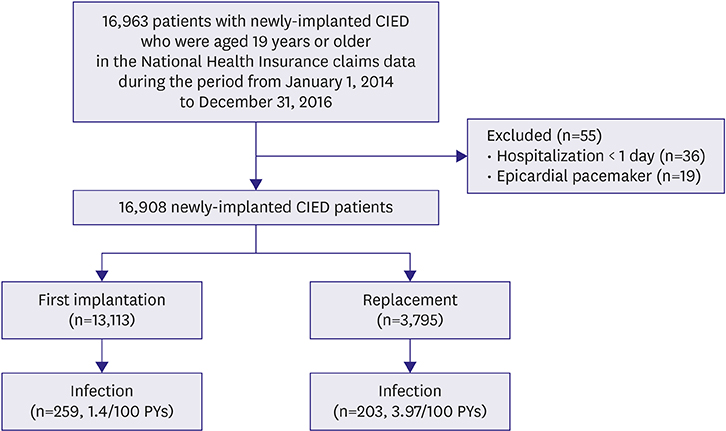
In the Health Insurance Review & Assessment Service (HIRA) database during the period from January 1, 2014 to December 31, 2016, we identified 16,908 patients with CIED implantation. CIED infection was defined as either: 1) Infection-related diagnosis code by the Korean Standard Classification of Diseases after any CIED procedure; or 2) CIED removal along with systemic infection.
RESULTS
The proportions of first implantation and replacement were 77.6% and 22.4%, respectively. During the follow-up period of 17.1±10.6 months, a total of 462 patients had CIED infection with incidence of 1.95 per 100 person-years with higher infection rate in replacement than first implantation (3.97 vs. 1.4 per 100 person-years, p<0.001). The average cost per person was US$ 16,584 (pacemaker, $13,736; implantable cardioverter defibrillator, $28,402; cardiac resynchronization therapy, $29,674). The risk factors of CIED infection were generator replacement (adjusted hazard ratio [aHR], 3.14; 95% confidence interval [CI], 2.60-3.78), diabetes mellitus (aHR, 1.94; 95% CI, 1.58-2.38), and congestive heart failure (aHR, 1.86; 95% CI, 1.51-2.28).
CONCLUSIONS
The rate of CIED infection in Korea was 1.95 per 100 person-years with average cost of US$ 16,584. The most important risk factor was generator replacement. This result suggests that generator replacement should be performed cautiously to avoid CIED infection.
MeSH Terms
Figure
Reference
-
1. Epstein AE, DiMarco JP, Ellenbogen KA, et al. 2012 ACCF/AHA/HRS focused update incorporated into the ACCF/AHA/HRS 2008 guidelines for device-based therapy of cardiac rhythm abnormalities: a report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines and the Heart Rhythm Society. Circulation. 2013; 127:e283–e352.2. Hunt SA, Abraham WT, Chin MH, et al. 2009 focused update incorporated into the acc/aha 2005 guidelines for the diagnosis and management of heart failure in adults a report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines developed in collaboration with the International Society for Heart and Lung Transplantation. J Am Coll Cardiol. 2009; 53:e1–e90.3. Baddour LM, Epstein AE, Erickson CC, et al. Update on cardiovascular implantable electronic device infections and their management: a scientific statement from the American Heart Association. Circulation. 2010; 121:458–477.4. Mangram AJ, Horan TC, Pearson ML, Silver LC, Jarvis WR. Hospital Infection Control Practices Advisory Committee. Guideline for prevention of surgical site infection, 1999. Infect Control Hosp Epidemiol. 1999; 20:250–278.
Article5. Mond HG, Irwin M, Ector H, Proclemer A. The world survey of cardiac pacing and cardioverter-defibrillators: calendar year 2005 an International Cardiac Pacing and Electrophysiology Society (ICPES) project. Pacing Clin Electrophysiol. 2008; 31:1202–1212.6. Voigt A, Shalaby A, Saba S. Continued rise in rates of cardiovascular implantable electronic device infections in the United States: temporal trends and causative insights. Pacing Clin Electrophysiol. 2010; 33:414–419.
Article7. Cabell CH, Heidenreich PA, Chu VH, et al. Increasing rates of cardiac device infections among Medicare beneficiaries: 1990–1999. Am Heart J. 2004; 147:582–586.
Article8. Eberhardt F, Bode F, Bonnemeier H, et al. Long term complications in single and dual chamber pacing are influenced by surgical experience and patient morbidity. Heart. 2005; 91:500–506.
Article9. Catanchin A, Murdock CJ, Athan E. Pacemaker infections: a 10-year experience. Heart Lung Circ. 2007; 16:434–439.
Article10. Lekkerkerker JC, van Nieuwkoop C, Trines SA, et al. Risk factors and time delay associated with cardiac device infections: Leiden device registry. Heart. 2009; 95:715–720.
Article11. Klug D, Balde M, Pavin D, et al. Risk factors related to infections of implanted pacemakers and cardioverter-defibrillators: results of a large prospective study. Circulation. 2007; 116:1349–1355.12. Kim TH, Yang PS, Uhm JS, et al. CHA2DS2-VASc score (congestive heart failure, hypertension, age ≥75 [doubled], diabetes mellitus, prior stroke or transient ischemic attack [doubled], vascular disease, age 65–74, female) for stroke in asian patients with atrial fibrillation: a Korean nationwide sample cohort study. Stroke. 2017; 48:1524–1530.13. Sohail MR, Uslan DZ, Khan AH, et al. Risk factor analysis of permanent pacemaker infection. Clin Infect Dis. 2007; 45:166–173.
Article14. McKibben L, Horan T, Tokars JI, et al. Guidance on public reporting of healthcare-associated infections: recommendations of the Healthcare Infection Control Practices Advisory Committee. Am J Infect Control. 2005; 33:217–226.
Article15. Hercé B, Nazeyrollas P, Lesaffre F, et al. Risk factors for infection of implantable cardiac devices: data from a registry of 2496 patients. Europace. 2013; 15:66–70.
Article16. Johansen JB, Jørgensen OD, Møller M, Arnsbo P, Mortensen PT, Nielsen JC. Infection after pacemaker implantation: infection rates and risk factors associated with infection in a population-based cohort study of 46299 consecutive patients. Eur Heart J. 2011; 32:991–998.
Article17. Uslan DZ, Sohail MR, St Sauver JL, et al. Permanent pacemaker and implantable cardioverter defibrillator infection: a population-based study. Arch Intern Med. 2007; 167:669–675.18. Uslan DZ. Infections of electrophysiologic cardiac devices. Expert Rev Med Devices. 2008; 5:183–195.
Article19. Marschall J, Hopkins-Broyles D, Jones M, Fraser VJ, Warren DK. Case-control study of surgical site infections associated with pacemakers and implantable cardioverter-defibrillators. Infect Control Hosp Epidemiol. 2007; 28:1299–1304.
Article20. Costea A, Rardon DP, Padanilam BJ, Fogel RI, Prystowsky EN. Complications associated with generator replacement in response to device advisories. J Cardiovasc Electrophysiol. 2008; 19:266–269.
Article21. Esposito S, Leone S. Prosthetic joint infections: microbiology, diagnosis, management and prevention. Int J Antimicrob Agents. 2008; 32:287–293.
Article22. Pichlmaier M, Marwitz V, Kühn C, et al. High prevalence of asymptomatic bacterial colonization of rhythm management devices. Europace. 2008; 10:1067–1072.
Article23. Anderson JM, Rodriguez A, Chang DT. Foreign body reaction to biomaterials. Semin Immunol. 2008; 20:86–100.
Article24. Subbiahdoss G, Kuijer R, Grijpma DW, van der Mei HC, Busscher HJ. Microbial biofilm growth vs. tissue integration: “the race for the surface” experimentally studied. Acta Biomater. 2009; 5:1399–1404.
Article25. Sohail MR, Henrikson CA, Braid-Forbes MJ, Forbes KF, Lerner DJ. Mortality and cost associated with cardiovascular implantable electronic device infections. Arch Intern Med. 2011; 171:1821–1828.
Article26. Voigt A, Shalaby A, Saba S. Rising rates of cardiac rhythm management device infections in the United States: 1996 through 2003. J Am Coll Cardiol. 2006; 48:590–591.
Article27. Klug D, Wallet F, Lacroix D, et al. Local symptoms at the site of pacemaker implantation indicate latent systemic infection. Heart. 2004; 90:882–886.
Article28. Klug D, Wallet F, Kacet S, Courcol RJ. Detailed bacteriologic tests to identify the origin of transvenous pacing system infections indicate a high prevalence of multiple organisms. Am Heart J. 2005; 149:322–328.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Hematoma Prevention Using Tachosil (Fibrin Sealant) Patch during Insertion of Cardiovascular Implantable Electronic Devices without Suspending Antithrombotics: Three Case Reports
- Risk Factors for Cardiac Implantable Electronic Device-Related Infections
- Rotational mechanical dilator sheaths for effective transvenous lead extraction
- Management of a Remnant Electrode in a Patient With Cardioverter-Defibrillator Infection After Refusal of Intravascular Electrode Removal
- Cardiac Rhythm Management Device Infections