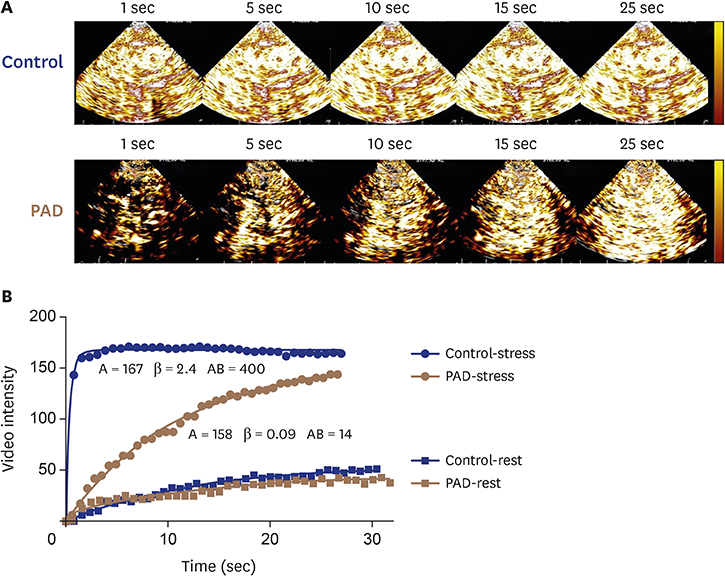
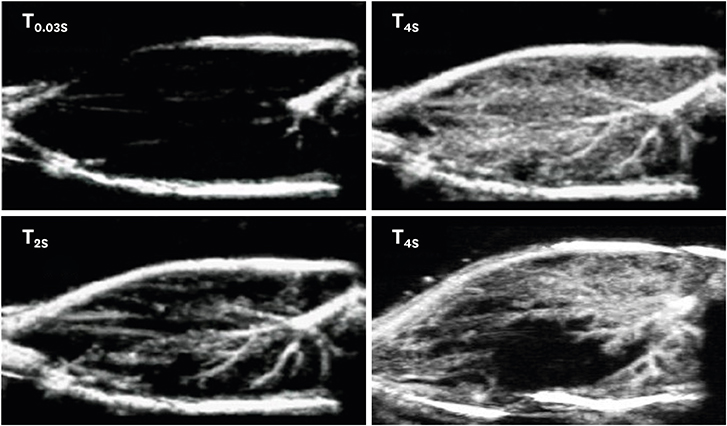
J Cardiovasc Imaging.
2019 Jul;27(3):163-177. 10.4250/jcvi.2019.27.e31.
Contrast Enhanced Ultrasound Perfusion Imaging in Skeletal Muscle
- Affiliations
-
- 1Knight Cardiovascular Institute, Oregon Health & Science University, Portland, OR, USA. davidsbr@ohsu.edu
- 2Veterans Affairs Portland Health Care System, Portland, OR, USA.
- KMID: 2456852
- DOI: http://doi.org/10.4250/jcvi.2019.27.e31
Abstract
- The ability to accurately evaluate skeletal muscle microvascular blood flow has broad clinical applications for understanding the regulation of skeletal muscle perfusion in health and disease states. Contrast-enhanced ultrasound (CEU) perfusion imaging, a technique originally developed to evaluate myocardial perfusion, is one of many techniques that have been applied to evaluate skeletal muscle perfusion. Among the advantages of CEU perfusion imaging of skeletal muscle is that it is rapid, safe and performed with equipment already present in most vascular medicine laboratories. The aim of this review is to discuss the use of CEU perfusion imaging in skeletal muscle. This article provides details of the protocols for CEU imaging in skeletal muscle, including two predominant methods for bolus and continuous infusion destruction-replenishment techniques. The importance of stress perfusion imaging will be highlighted, including a discussion of the methods used to produce hyperemic skeletal muscle blood flow. A broad overview of the disease states that have been studied in humans using CEU perfusion imaging of skeletal muscle will be presented including: (1) peripheral arterial disease; (2) sickle cell disease; (3) diabetes; and (4) heart failure. Finally, future applications of CEU imaging in skeletal muscle including therapeutic CEU imaging will be discussed along with technological developments needed to advance the field.
MeSH Terms
Figure
Reference
-
1. Joyner MJ, Casey DP. Regulation of increased blood flow (hyperemia) to muscles during exercise: a hierarchy of competing physiological needs. Physiol Rev. 2015; 95:549–601.
Article2. Calbet JA, Lundby C. Skeletal muscle vasodilatation during maximal exercise in health and disease. J Physiol. 2012; 590:6285–6296.
Article3. Gliemann L, Mortensen SP, Hellsten Y. Methods for the determination of skeletal muscle blood flow: development, strengths and limitations. Eur J Appl Physiol. 2018; 118:1081–1094.
Article4. Dunford EC, Au JS, Devries MC, Phillips SM, MacDonald MJ. Cardiovascular aging and the microcirculation of skeletal muscle: using contrast-enhanced ultrasound. Am J Physiol Heart Circ Physiol. 2018; 315:H1194–H1199.
Article5. Lindner JR, Song J, Jayaweera AR, Sklenar J, Kaul S. Microvascular rheology of Definity microbubbles after intra-arterial and intravenous administration. J Am Soc Echocardiogr. 2002; 15:396–403.
Article6. Gramiak R, Shah PM. Echocardiography of the aortic root. Invest Radiol. 1968; 3:356–366.
Article7. Rafter P, Phillips P, Vannan MA. Imaging technologies and techniques. Cardiol Clin. 2004; 22:181–197.
Article8. Burns PN, Wilson SR. Microbubble contrast for radiological imaging: 1. Principles. Ultrasound Q. 2006; 22:5–13.9. Wei K, Jayaweera AR, Firoozan S, Linka A, Skyba DM, Kaul S. Quantification of myocardial blood flow with ultrasound-induced destruction of microbubbles administered as a constant venous infusion. Circulation. 1998; 97:473–483.
Article10. Ryu JC, Davidson BP, Xie A, et al. Molecular imaging of the paracrine proangiogenic effects of progenitor cell therapy in limb ischemia. Circulation. 2013; 127:710–719.
Article11. Seol SH, Davidson BP, Belcik JT, et al. Real-time contrast ultrasound muscle perfusion imaging with intermediate-power imaging coupled with acoustically durable microbubbles. J Am Soc Echocardiogr. 2015; 28:718–26.e2.
Article12. Davidson BP, Hodovan J, Belcik JT, et al. Rest-stress limb perfusion imaging in humans with contrast ultrasound using intermediate-power imaging and microbubbles resistant to inertial cavitation. J Am Soc Echocardiogr. 2017; 30:503–510.e1.
Article13. Carr CL, Qi Y, Davidson B, et al. Dysregulated selectin expression and monocyte recruitment during ischemia-related vascular remodeling in diabetes mellitus. Arterioscler Thromb Vasc Biol. 2011; 31:2526–2533.
Article14. Wu MD, Belcik JT, Qi Y, et al. Abnormal regulation of microvascular tone in a murine model of sickle cell disease assessed by contrast ultrasound. J Am Soc Echocardiogr. 2015; 28:1122–1128.15. Shim CY, Kim S, Chadderdon S, et al. Epoxyeicosatrienoic acids mediate insulin-mediated augmentation in skeletal muscle perfusion and blood volume. Am J Physiol Endocrinol Metab. 2014; 307:E1097–104.
Article16. Davidson BP, Belcik JT, Mott BH, Landry G, Lindner JR. Quantification of residual limb skeletal muscle perfusion with contrast-enhanced ultrasound during application of a focal junctional tourniquet. J Vasc Surg. 2016; 63:148–153.
Article17. Duerschmied D, Olson L, Olschewski M, et al. Contrast ultrasound perfusion imaging of lower extremities in peripheral arterial disease: a novel diagnostic method. Eur Heart J. 2006; 27:310–315.
Article18. Duerschmied D, Zhou Q, Rink E, et al. Simplified contrast ultrasound accurately reveals muscle perfusion deficits and reflects collateralization in PAD. Atherosclerosis. 2009; 202:505–512.
Article19. Duerschmied D, Maletzki P, Freund G, Olschewski M, Bode C, Hehrlein C. Success of arterial revascularization determined by contrast ultrasound muscle perfusion imaging. J Vasc Surg. 2010; 52:1531–1536.
Article20. Palmowski M, Lederle W, Gaetjens J, et al. Comparison of conventional time-intensity curves vs. maximum intensity over time for post-processing of dynamic contrast-enhanced ultrasound. Eur J Radiol. 2010; 75:e149–e153.
Article21. Belcik JT, Qi Y, Kaufmann BA, et al. Cardiovascular and systemic microvascular effects of anti-vascular endothelial growth factor therapy for cancer. J Am Coll Cardiol. 2012; 60:618–625.22. Heinonen I, Kemppainen J, Kaskinoro K, et al. Comparison of exogenous adenosine and voluntary exercise on human skeletal muscle perfusion and perfusion heterogeneity. J Appl Physiol (1985). 2010; 108:378–386.
Article23. Davidson BP, Belcik JT, Landry G, Linden J, Lindner JR. Exercise versus vasodilator stress limb perfusion imaging for the assessment of peripheral artery disease. Echocardiography. 2017; 34:1187–1194.
Article24. Lindner JR, Womack L, Barrett EJ, et al. Limb stress-rest perfusion imaging with contrast ultrasound for the assessment of peripheral arterial disease severity. JACC Cardiovasc Imaging. 2008; 1:343–350.
Article25. Womack L, Peters D, Barrett EJ, Kaul S, Price W, Lindner JR. Abnormal skeletal muscle capillary recruitment during exercise in patients with type 2 diabetes mellitus and microvascular complications. J Am Coll Cardiol. 2009; 53:2175–2183.
Article26. Leong-Poi H, Swales J, Jayaweera AR, Bin JP, Kaul S, Lindner JR. Effect of microbubble exposure to ultrasound on quantitation of myocardial perfusion. Echocardiography. 2005; 22:503–509.
Article27. Amarteifio E, Weber MA, Wormsbecher S, et al. Dynamic contrast-enhanced ultrasound for assessment of skeletal muscle microcirculation in peripheral arterial disease. Invest Radiol. 2011; 46:504–508.
Article28. Krix M, Krakowski-Roosen H, Amarteifio E, et al. Comparison of transient arterial occlusion and muscle exercise provocation for assessment of perfusion reserve in skeletal muscle with real-time contrast-enhanced ultrasound. Eur J Radiol. 2011; 78:419–424.
Article29. Krix M, Weber MA, Kauczor HU, Delorme S, Krakowski-Roosen H. Changes in the micro-circulation of skeletal muscle due to varied isometric exercise assessed by contrast-enhanced ultrasound. Eur J Radiol. 2010; 76:110–116.
Article30. Klabunde RE, Laughlin MH, Armstrong RB. Systemic adenosine deaminase administration does not reduce active hyperemia in running rats. J Appl Physiol (1985). 1988; 64:108–114.
Article31. Bragadeesh T, Sari I, Pascotto M, Micari A, Kaul S, Lindner JR. Detection of peripheral vascular stenosis by assessing skeletal muscle flow reserve. J Am Coll Cardiol. 2005; 45:780–785.
Article32. Amarteifio E, Wormsbecher S, Krix M, et al. Dynamic contrast-enhanced ultrasound and transient arterial occlusion for quantification of arterial perfusion reserve in peripheral arterial disease. Eur J Radiol. 2012; 81:3332–3338.
Article33. Amarteifio E, Krix M, Wormsbecher S, et al. Dynamic contrast-enhanced ultrasound for assessment of therapy effects on skeletal muscle microcirculation in peripheral arterial disease: pilot study. Eur J Radiol. 2013; 82:640–646.
Article34. Thomas KN, Cotter JD, Lucas SJ, Hill BG, van Rij AM. Reliability of contrast-enhanced ultrasound for the assessment of muscle perfusion in health and peripheral arterial disease. Ultrasound Med Biol. 2015; 41:26–34.
Article35. Goh V, Halligan S, Hugill JA, Bartram CI. Quantitative assessment of tissue perfusion using MDCT: comparison of colorectal cancer and skeletal muscle measurement reproducibility. AJR Am J Roentgenol. 2006; 187:164–169.
Article36. Ware RE, de Montalembert M, Tshilolo L, Abboud MR. Sickle cell disease. Lancet. 2017; 390:311–323.
Article37. Sachdev V, Sidenko S, Wu MD, et al. Skeletal and myocardial microvascular blood flow in hydroxycarbamide-treated patients with sickle cell disease. Br J Haematol. 2017; 179:648–656.
Article38. Rim SJ, Leong-Poi H, Lindner JR, Wei K, Fisher NG, Kaul S. Decrease in coronary blood flow reserve during hyperlipidemia is secondary to an increase in blood viscosity. Circulation. 2001; 104:2704–2709.
Article39. Lindner JR, Widlansky M, Wu MD, et al. Contrast-enhanced ultrasound detects differences in microvascular blood flow in adults with sickle cell disease administered regadenoson. Blood. 2014; 124:2705.
Article40. Wu MD, Moccetti F, Brown E, et al. Lipoprotein apheresis acutely reverses coronary microvascular dysfunction in patients with severe hypercholesterolemia. JACC Cardiovasc Imaging. 2018; 06. 19. E-pub ahead of print. DOI: 10.1016/j.jcmg.2018.05.001.41. Chan A, Barrett EJ, Anderson SM, Kovatchev BP, Breton MD. Muscle microvascular recruitment predicts insulin sensitivity in middle-aged patients with type 1 diabetes mellitus. Diabetologia. 2012; 55:729–736.
Article42. Russell RD, Hu D, Greenaway T, et al. Skeletal muscle microvascular-linked improvements in glycemic control from resistance training in individuals with type 2 diabetes. Diabetes Care. 2017; 40:1256–1263.
Article43. Eggleston EM, Jahn LA, Barrett EJ. Hyperinsulinemia rapidly increases human muscle microvascular perfusion but fails to increase muscle insulin clearance: evidence that a saturable process mediates muscle insulin uptake. Diabetes. 2007; 56:2958–2963.
Article44. Sjøberg KA, Frøsig C, Kjøbsted R, et al. Exercise increases human skeletal muscle insulin sensitivity via coordinated increases in microvascular perfusion and molecular signaling. Diabetes. 2017; 66:1501–1510.
Article45. Walsh LK, Ghiarone T, Olver TD, et al. Increased endothelial shear stress improves insulin-stimulated vasodilatation in skeletal muscle. J Physiol. 2019; 597:57–69.
Article46. Manetos C, Dimopoulos S, Tzanis G, et al. Skeletal muscle microcirculatory abnormalities are associated with exercise intolerance, ventilatory inefficiency, and impaired autonomic control in heart failure. J Heart Lung Transplant. 2011; 30:1403–1408.
Article47. Haykowsky MJ, Tomczak CR, Scott JM, Paterson DI, Kitzman DW. Determinants of exercise intolerance in patients with heart failure and reduced or preserved ejection fraction. J Appl Physiol (1985). 2015; 119:739–744.
Article48. Poole DC, Richardson RS, Haykowsky MJ, Hirai DM, Musch TI. Exercise limitations in heart failure with reduced and preserved ejection fraction. J Appl Physiol (1985). 2018; 124:208–224.
Article49. Wilson JR, Mancini DM, McCully K, Ferraro N, Lanoce V, Chance B. Noninvasive detection of skeletal muscle underperfusion with near-infrared spectroscopy in patients with heart failure. Circulation. 1989; 80:1668–1674.
Article50. Barrett-O'Keefe Z, Lee JF, Berbert A, et al. Hemodynamic responses to small muscle mass exercise in heart failure patients with reduced ejection fraction. Am J Physiol Heart Circ Physiol. 2014; 307:H1512–20.51. Jablonowski LJ, Stanczak M, Machado P, Fitzgerald K, Reeves GR, Forsberg F. Contrast-enhanced ultrasound evaluation of skeletal muscle perfusion in response to left ventricular assist device (LVAD) therapy. In : 2017 IEEE International Ultrasonics Symposium (IUS); Sep 6-9, 2017; Washington, DC. p. 1–3.52. Reeves GR, Jablonowski LJ, Stanczak M, Machado P, Fitzgerald K, Forsberg F. Contrast enhanced ultrasound evaluation of skeletal muscle perfusion in response to left ventricular assist device therapy. J Heart Lung Transplant. 2019; 38:S453.53. Belcik JT, Mott BH, Xie A, et al. Augmentation of limb perfusion and reversal of tissue ischemia produced by ultrasound-mediated microbubble cavitation. Circ Cardiovasc Imaging. 2015; 8:e002979.
Article54. Belcik JT, Davidson BP, Xie A, et al. Augmentation of muscle blood flow by ultrasound cavitation is mediated by ATP and purinergic signaling. Circulation. 2017; 135:1240–1252.
Article55. Chappell JC, Price RJ. Targeted therapeutic applications of acoustically active microspheres in the microcirculation. Microcirculation. 2006; 13:57–70.
Article56. Castle J, Feinstein SB. Drug and gene delivery using sonoporation for cardiovascular disease. Adv Exp Med Biol. 2016; 880:331–338.
Article57. Unger E, Porter T, Lindner J, Grayburn P. Cardiovascular drug delivery with ultrasound and microbubbles. Adv Drug Deliv Rev. 2014; 72:110–126.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- A Primer on the Methods and Applications for Contrast Echocardiography in Clinical Imaging
- Contrast-enhanced ultrasound of hepatocellular carcinoma: where do we stand?
- Future Applications of Contrast Ultrasound
- Contrast Enhanced US in the Abdomen
- Up-to-date Doppler techniques for breast tumor vascularity: superb microvascular imaging and contrast-enhanced ultrasound