Ann Dermatol.
2019 Oct;31(5):502-510. 10.5021/ad.2019.31.5.502.
Melandrium firmum Extract Promotes Hair Growth by Modulating 5α-Reductase Activity and Gene Expression in C57BL/6J Mice
- Affiliations
-
- 1Department of Food Science and Engineering, Jinzhou Medical University, Jinzhou, China. xhzhang107@126.com
- 2Department of Food Science and Nutrition, Hallym University, Chuncheon, Korea. limss@hallym.ac.kr
- KMID: 2456226
- DOI: http://doi.org/10.5021/ad.2019.31.5.502
Abstract
- BACKGROUND
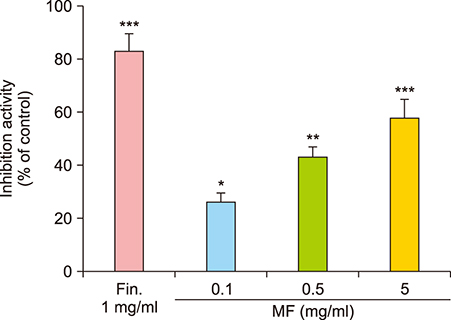
In our preliminary study, we screened for their potential to inhibit 5α-reductase, and Melandrium firmum (MF) extract showed the most potent activity as confirmed by high-performance liquid chromatography (HPLC).
OBJECTIVE
This study aimed to investigate the effects of MF extract on 5α-reductase activity and its mechanisms of action in the prevention or treatment of androgenetic alopecia.
METHODS
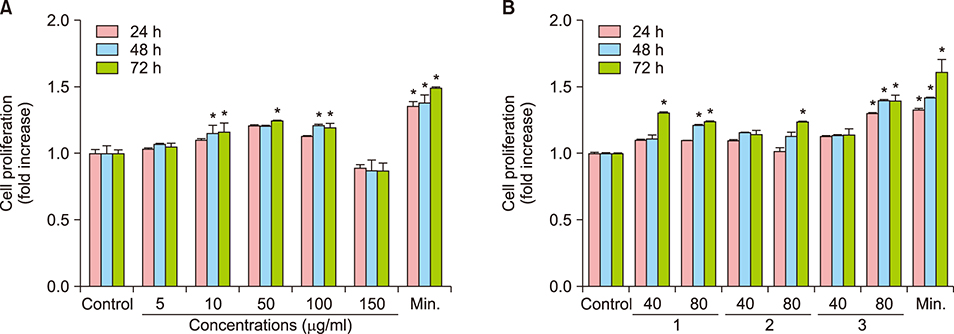
HPLC was used to measure 5α-reductase activity. The hair growth-promoting effect of MF extract in the shaved dorsal skin of C57BL/6J mice was studied for 30 days. Hair follicles were examined by histological examination. Protein and mRNA levels of growth factors involved in hair growth were determined by western blotting, and reverse transcription-polymerase chain reaction (RT-PCR) and qPCR, respectively. Cell proliferation was measured by (3-(4, 5-dimethylthiazol-2-yl)-5-(3-carboxymethoxyphenyl)-2-(4-sulfophenyl)-2H-tetrazolium) assay.
RESULTS
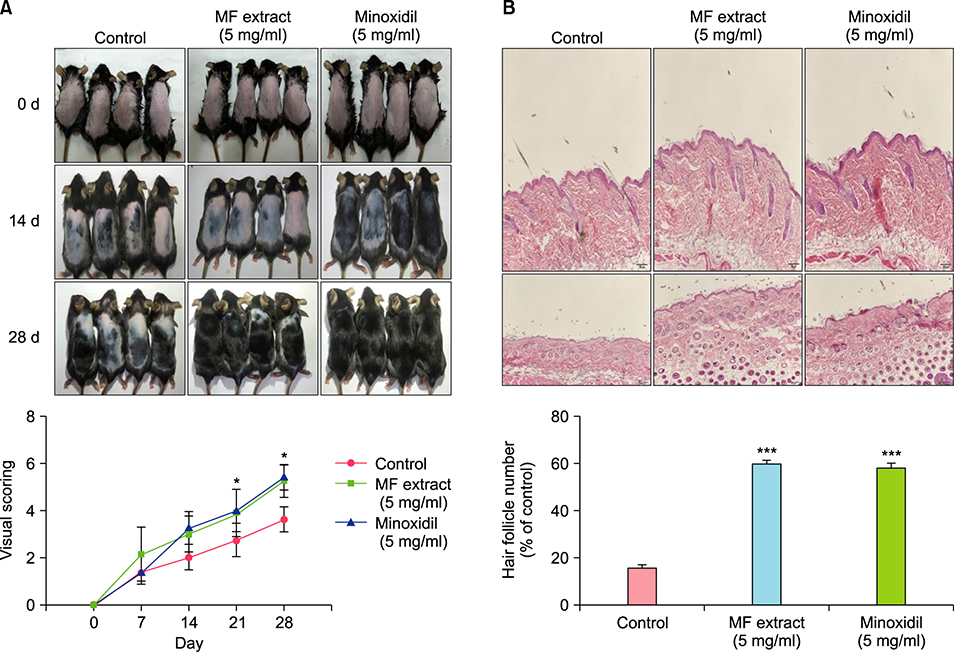
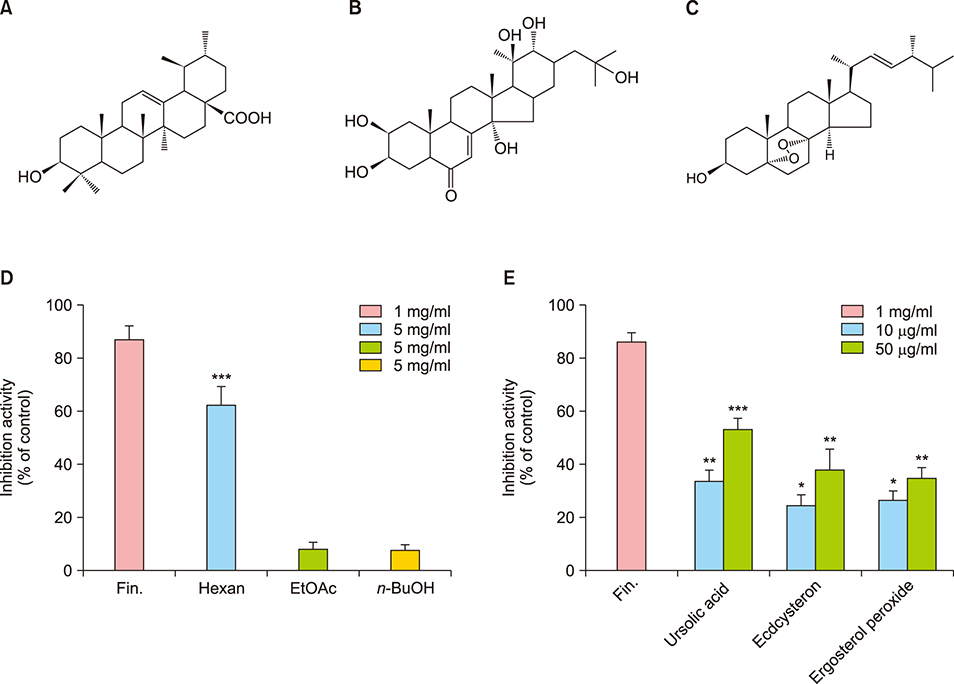
MF extract at 0.5 mg/ml showed 43.5% inhibition of 5α-reductase. MF extract promoted hair growth by inducing anagen phase reflected by skin color, hair density, and the number and size of hair follicles. It not only reduces the expression of transforming growth factor-beta 1 (TGF-β1) and Dickkopf-1 (DKK-1), but also markedly upregulated insulin-like growth factor 1 and keratinocyte growth factor in the dorsal dermal tissue. Ursolic acid, ecdcysteron, and ergosterol peroxide were identified as active constituents by activity-guided fractionation to inhibit 5α-reductase. They decreased the gene expression of TGF-β1 and DKK-1 in human hair dermal papilla cells.
CONCLUSION
In summary, these finding indicate that MF extract might be a good drug candidate for hair growth promotion.
MeSH Terms
-
Alopecia
Animals
Blotting, Western
Cell Proliferation
Chromatography, High Pressure Liquid
Chromatography, Liquid
Ergosterol
Fibroblast Growth Factor 7
Gene Expression*
Hair Follicle
Hair*
Humans
Intercellular Signaling Peptides and Proteins
Mice*
RNA, Messenger
Skin
Skin Pigmentation
Ergosterol
Fibroblast Growth Factor 7
Intercellular Signaling Peptides and Proteins
RNA, Messenger
Figure
Reference
-
1. Kerscher M, Williams S, Dubertret L. Cosmetic dermatology and skin care. Eur J Dermatol. 2007; 17:180–182.2. Paik JH, Yoon JB, Sim WY, Kim BS, Kim NI. The prevalence and types of androgenetic alopecia in Korean men and women. Br J Dermatol. 2001; 145:95–99.
Article3. Kang JI, Kim SC, Han SC, Hong HJ, Jeon YJ, Kim B, et al. Hair-loss preventing effect of Grateloupia elliptica. Biomol Ther (Seoul). 2012; 20:118–124.
Article4. Ellis JA, Sinclair R, Harrap SB. Androgenetic alopecia: pathogenesis and potential for therapy. Expert Rev Mol Med. 2002; 4:1–11.
Article5. Hadshiew IM, Foitzik K, Arck PC, Paus R. Burden of hair loss: stress and the underestimated psychosocial impact of telogen effluvium and androgenetic alopecia. J Invest Dermatol. 2004; 123:455–457.
Article6. Burton JL, Marshall A. Hypertrichosis due to minoxidil. Br J Dermatol. 1979; 101:593–595.
Article7. Kaufman KD, Olsen EA, Whiting D, Savin R, DeVillez R, Bergfeld W, et al. Finasteride in the treatment of men with androgenetic alopecia. Finasteride Male Pattern Hair Loss Study Group. J Am Acad Dermatol. 1998; 39:578–589.8. Shorter K, Farjo NP, Picksley SM, Randall VA. Human hair follicles contain two forms of ATP-sensitive potassium channels, only one of which is sensitive to minoxidil. FASEB J. 2008; 22:1725–1736.
Article9. Murata K, Noguchi K, Kondo M, Onishi M, Watanabe N, Okamura K, et al. Promotion of hair growth by Rosmarinus officinalis leaf extract. Phytother Res. 2013; 27:212–217.10. Patel S, Sharma V, Chauhan NS, Thakur M, Dixit VK. Hair growth: focus on herbal therapeutic agent. Curr Drug Discov Technol. 2015; 12:21–42.
Article11. Kang JI, Kim SC, Hyun JH, Kang JH, Park DB, Lee YJ, et al. Promotion effect of Schisandra nigra on the growth of hair. Eur J Dermatol. 2009; 19:119–125.12. Park HJ, Zhang N, Park DK. Topical application of Polygonum multiflorum extract induces hair growth of resting hair follicles through upregulating Shh and β-catenin expression in C57BL/6 mice. J Ethnopharmacol. 2011; 135:369–375.
Article13. Rahman MA, Yang H, Lim SS, Huh SO. Apoptotic effects of Melandryum firmum root extracts in human SH-SY5Y neuroblastoma cells. Exp Neurobiol. 2013; 22:208–213.
Article14. Zheng MS, Hwang NK, Kim DH, Moon TC, Son JK, Chang HW. Chemical constituents of Melandrium firmum Rohrbach and their anti-inflammatory activity. Arch Pharm Res. 2008; 31:318–322.
Article15. Matsuda H, Sato N, Yamazaki M, Naruto S, Kubo M. Testosterone 5alpha-reductase inhibitory active constituents from Anemarrhenae Rhizoma. Biol Pharm Bull. 2001; 24:586–587.
Article16. Müller-Röver S, Handjiski B, van der, Eichmüller S, Foitzik K, McKay IA, et al. A comprehensive guide for the accurate classification of murine hair follicles in distinct hair cycle stages. J Invest Dermatol. 2001; 117:3–15.
Article17. Tan JJY, Pan J, Sun L, Zhang J, Wu C, Kang L. Bioactives in Chinese proprietary medicine modulates 5α-reductase activity and gene expression associated with androgenetic alopecia. Front Pharmacol. 2017; 8:194.
Article18. Inui S, Fukuzato Y, Nakajima T, Yoshikawa K, Itami S. Androgen-inducible TGF-beta1 from balding dermal papilla cells inhibits epithelial cell growth: a clue to understand paradoxical effects of androgen on human hair growth. FASEB J. 2002; 16:1967–1969.
Article19. Inui S, Fukuzato Y, Nakajima T, Yoshikawa K, Itami S. Identification of androgen-inducible TGF-beta1 derived from dermal papilla cells as a key mediator in androgenetic alopecia. J Investig Dermatol Symp Proc. 2003; 8:69–71.
Article20. Kwack MH, Sung YK, Chung EJ, Im SU, Ahn JS, Kim MK, et al. Dihydrotestosterone-inducible dickkopf 1 from balding dermal papilla cells causes apoptosis in follicular keratinocytes. J Invest Dermatol. 2008; 128:262–269.
Article21. Andl T, Reddy ST, Gaddapara T, Millar SE. WNT signals are required for the initiation of hair follicle development. Dev Cell. 2002; 2:643–653.
Article22. Zhao J, Harada N, Kurihara H, Nakagata N, Okajima K. Dietary isoflavone increases insulin-like growth factor-I production, thereby promoting hair growth in mice. J Nutr Biochem. 2011; 22:227–233.
Article23. Rajendran RL, Gangadaran P, Bak SS, Oh JM, Kalimuthu S, Lee HW, et al. Extracellular vesicles derived from MSCs activates dermal papilla cell in vitro and promotes hair follicle conversion from telogen to anagen in mice. Sci Rep. 2017; 7:15560.
Article24. Werner S, Smola H, Liao X, Longaker MT, Krieg T, Hofschneider PH, et al. The function of KGF in morphogenesis of epithelium and reepithelialization of wounds. Science. 1994; 266:819–822.
Article25. Jena AK, Vasisht K, Sharma N, Kaur R, Dhingra MS, Karan M. Amelioration of testosterone induced benign prostatic hyperplasia by Prunus species. J Ethnopharmacol. 2016; 190:33–45.
Article26. Christensen KB, Petersen RK, Kristiansen K, Christensen LP. Identification of bioactive compounds from flowers of black elder (Sambucus nigra L.) that activate the human peroxisome proliferator-activated receptor (PPAR) gamma. Phytother Res. 2010; 24 Suppl 2:S129–S132.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Over-expression of myosin7A in cochlear hair cells of circling mice
- Effect of Seaweed Extract on Hair Growth Promotion in Experimental Study of C57BL/6 Mice
- Promotional effects of Sargassum fusiforme fractions on hair growth via in vitro and in vivo models
- Hair growth promoting effects of emodin in telogenic C57BL/6 mice
- RE-ORGA, a Korean Herb Extract, Can Prevent Hair Loss Induced by Dihydrotestosterone in Human Dermal Papilla Cells