J Nutr Health.
2018 Dec;51(6):498-506. 10.4163/jnh.2018.51.6.498.
Protective effect of lycopene against cytokine-induced β-cell apoptosis in INS-1 cells
- Affiliations
-
- 1Department of Food and Nutrition, Eulji University, Seongnam, Gyeonggi 13135, Korea. ysoh@eulji.ac.kr
- 2Lee Gil Ya Cancer and Diabetes Institute, Department of Molecular Medicine, Gachon University, Incheon 21999, Korea.
- 3College of Pharmacy, Gachon University, Incheon 21936, Korea.
- 4Gachon Gil Medical Center, Incheon 21565, Korea.
- KMID: 2455838
- DOI: http://doi.org/10.4163/jnh.2018.51.6.498
Abstract
- PURPOSE
Lycopene, a carotenoid with anti-oxidant properties, occurs naturally in tomatoes and pink grapefruit. Although the beneficial effects of lycopene on various disorders have been established, little attention has been paid to the possible anti-diabetic effects of lycopene focusing on β-cells. Therefore, this study investigated the potential of lycopene to protect β-cells against apoptosis induced by a cytokine mixture.
METHODS
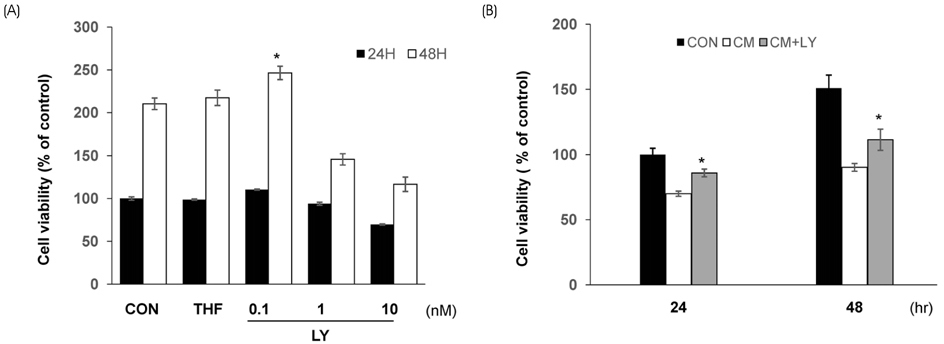
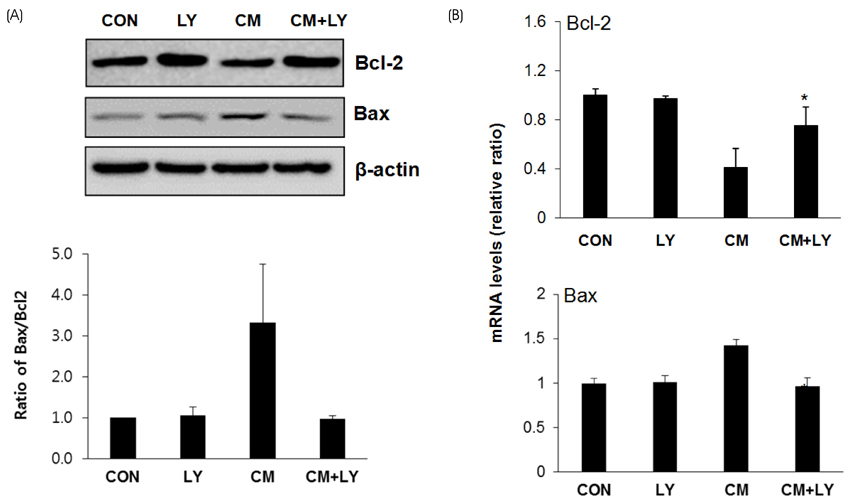
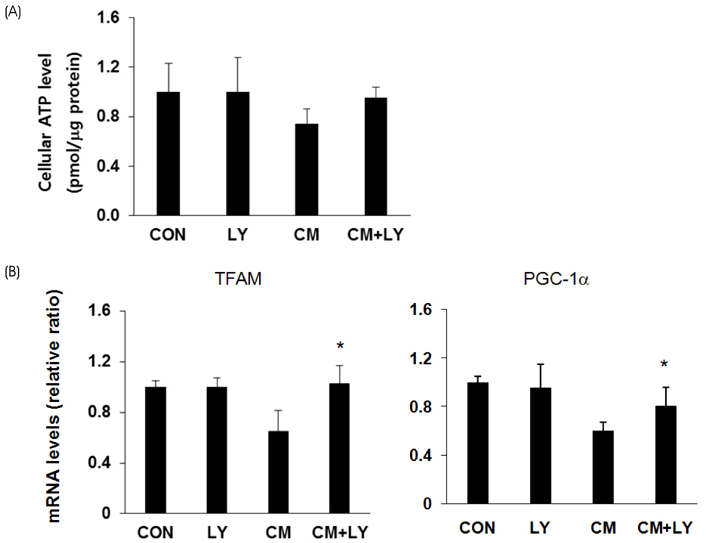
For toxicity experiments, the cells were treated with 0.1 ~ 10 nM of lycopene, and the cell viability in INS-1 cells (a rat β-cell line) was measured using a MTT assay. To induce cytokine toxicity, the cells were treated with a cytokine mixture (20 ng/mL of TNFα+20 ng/mL of IL-1β) for 24 h, and the effects of lycopene (0.1 nM) on the cytokine toxicity were measured using the MTT assay. The expression levels of the apoptotic proteins were analyzed by Western blotting, and the level of intracellular reactive oxidative stress (ROS) was monitored using a DCFDA fluorescent probe. The intracellular ATP levels were determined using a luminescence kit, and mRNA expression of the genes coding for anti-oxidative stress response and mitochondrial function were analyzed by quantitative reverse-transcriptase PCR.
RESULTS
Exposure of INS-1 cells to 0.1 nM of lycopene increased the cell viability significantly, and protected the cells from cytokine-induced death. Lycopene upregulated the mRNA and protein expression of B-cell lymphoma-2 (Bcl-2) and reduced the expression of the Bcl-2 associated X (Bax) protein. Lycopene inhibited apoptotic signaling via a reduction of the ROS, and this effect correlated with the upregulation of anti-oxidative stress response genes, such as GCLC, NQO1, and HO-1. Lycopene increased the mRNA expression of mitochondrial function-related genes and increased the cellular ATP level.
CONCLUSION
These results suggest that lycopene reduces the level of oxidative stress and improves the mitochondrial function, contributing to the prevention of cytokine-induced β-cell apoptosis. Therefore, lycopene could potentially serve as a preventive and therapeutic agent for the treatment of type 2 diabetes.
Keyword
MeSH Terms
Figure
Reference
-
1. Rhodes CJ. Type 2 diabetes-a matter of beta-cell life and death? Science. 2005; 307(5708):380–384.2. Shaw JE, Sicree RA, Zimmet PZ. Global estimates of the prevalence of diabetes for 2010 and 2030. Diabetes Res Clin Pract. 2010; 87(1):4–14.
Article3. Kahn SE, Hull RL, Utzschneider KM. Mechanisms linking obesity to insulin resistance and type 2 diabetes. Nature. 2006; 444(7121):840–846.
Article4. Baynes JW, Thorpe SR. Role of oxidative stress in diabetic complications: a new perspective on an old paradigm. Diabetes. 1999; 48(1):1–9.
Article5. Tiedge M, Lortz S, Drinkgern J, Lenzen S. Relation between antioxidant enzyme gene expression and antioxidative defense status of insulin-producing cells. Diabetes. 1997; 46(11):1733–1742.
Article6. Gorogawa Si, Kajimoto Y, Umayahara Y, Kaneto H, Watada H, Kuroda A, Kawamori D, Yasuda T, Matsuhisa M, Yamasaki Y, Hori M. Probucol preserves pancreatic beta-cell function through reduction of oxidative stress in type 2 diabetes. Diabetes Res Clin Pract. 2002; 57(1):1–10.7. Robertson RP, Harmon JS. Pancreatic islet beta-cell and oxidative stress: the importance of glutathione peroxidase. FEBS Lett. 2007; 581(19):3743–3748.8. Stahl W, Sies H. Lycopene: a biologically important carotenoid for humans? Arch Biochem Biophys. 1996; 336(1):1–9.
Article9. Ali MM, Agha FG. Amelioration of streptozotocin-induced diabetes mellitus, oxidative stress and dyslipidemia in rats by tomato extract lycopene. Scand J Clin Lab Invest. 2009; 69(3):371–379.
Article10. Wang L, Liu S, Manson JE, Gaziano JM, Buring JE, Sesso HD. The consumption of lycopene and tomato-based food products is not associated with the risk of type 2 diabetes in women. J Nutr. 2006; 136(3):620–625.
Article11. Oh YS, Lee YJ, Park EY, Jun HS. Interleukin-6 treatment induces beta-cell apoptosis via STAT-3-mediated nitric oxide production. Diabetes Metab Res Rev. 2011; 27(8):813–819.
Article12. Mosmann T. Rapid colorimetric assay for cellular growth and survival: application to proliferation and cytotoxicity assays. J Immunol Methods. 1983; 65(1-2):55–63.
Article13. Antonsson B, Martinou JC. The Bcl-2 protein family. Exp Cell Res. 2000; 256(1):50–57.
Article14. Jürgensmeier JM, Xie Z, Deveraux Q, Ellerby L, Bredesen D, Reed JC. Bax directly induces release of cytochrome c from isolated mitochondria. Proc Natl Acad Sci U S A. 1998; 95(9):4997–5002.15. Padgett LE, Broniowska KA, Hansen PA, Corbett JA, Tse HM. The role of reactive oxygen species and proinflammatory cytokines in type 1 diabetes pathogenesis. Ann N Y Acad Sci. 2013; 1281(1):16–35.
Article16. Cho YM, Park KS, Lee HK. Genetic factors related to mitochondrial function and risk of diabetes mellitus. Diabetes Res Clin Pract. 2007; 77:3 Suppl 1. S172–S177.
Article17. Lowell BB, Shulman GI. Mitochondrial dysfunction and type 2 diabetes. Science. 2005; 307(5708):384–387.
Article18. Kim EK, Kwon KB, Han MJ, Song MY, Lee JH, Lv N, Choi KB, Ryu DG, Kim KS, Park JW, Park BH. Inhibitory effect of Artemisia capillaris extract on cytokine-induced nitric oxide formation and cytotoxicity of RINm5F cells. Int J Mol Med. 2007; 19(3):535–540.
Article19. Mandrup-Poulsen T. Apoptotic signal transduction pathways in diabetes. Biochem Pharmacol. 2003; 66(8):1433–1440.
Article20. Cnop M, Welsh N, Jonas JC, Jörns A, Lenzen S, Eizirik DL. Mechanisms of pancreatic beta-cell death in type 1 and type 2 diabetes: many differences, few similarities. Diabetes. 2005; 54:Suppl 2. S97–S107.21. Hanafusa T, Imagawa A. Insulitis in human type 1 diabetes. Ann N Y Acad Sci. 2008; 1150(1):297–299.
Article22. Kim EK, Kwon KB, Song MY, Seo SW, Park SJ, Ka SO, Na L, Kim KA, Ryu DG, So HS, Park R, Park JW, Park BH. Genistein protects pancreatic beta cells against cytokine-mediated toxicity. Mol Cell Endocrinol. 2007; 278(1-2):18–28.23. Oh YS, Jun HS. Effects of glucagon-like peptide-1 on oxidative stress and Nrf2 signaling. Int J Mol Sci. 2017; 19(1):E26.
Article24. Holmström KM, Finkel T. Cellular mechanisms and physiological consequences of redox-dependent signalling. Nat Rev Mol Cell Biol. 2014; 15(6):411–421.
Article25. Zhu J, Wang CG, Xu YG. Lycopene attenuates endothelial dysfunction in streptozotocin-induced diabetic rats by reducing oxidative stress. Pharm Biol. 2011; 49(11):1144–1149.
Article26. Huang C, Gan D, Fan C, Wen C, Li A, Li Q, Zhao J, Wang Z, Zhu L, Lu D. The secretion from neural stem cells pretreated with lycopene protects against tert-butyl hydroperoxide-induced neuron oxidative damage. Oxid Med Cell Longev. 2018; 2018:5490218.
Article27. Larsson NG, Wang J, Wilhelmsson H, Oldfors A, Rustin P, Lewandoski M, Barsh GS, Clayton DA. Mitochondrial transcription factor A is necessary for mtDNA maintenance and embryogenesis in mice. Nat Genet. 1998; 18(3):231–236.28. Zhang P, Liu C, Zhang C, Zhang Y, Shen P, Zhang J, Zhang CY. Free fatty acids increase PGC-1alpha expression in isolated rat islets. FEBS Lett. 2005; 579(6):1446–1452.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Protective effect of cyanidin-3-O-glucoside against tacrolimus-induced pancreatic beta cell dysfunction
- Effect of β-carotene on Cell Growth Inhibition of KB Human Oral Cancer Cells
- The Effects of Exendin-4 on IRS-2 Expression and Phosphorylation in INS-1 Cells
- Padina arborescens extract protects high glucose-induced apoptosis in pancreatic beta cells by reducing oxidative stress
- Mitogenic Effects and Signaling Pathway of Insulin-Like Growth Factor-I (IGF-I) in the Rat Beta Cell Line (INS-1)