J Nutr Health.
2018 Dec;51(6):489-497. 10.4163/jnh.2018.51.6.489.
Effects of high-fat diet induced obesity on tissue zinc concentrations and zinc transporter expressions in mice
- Affiliations
-
- 1Department of Food & Nutrition, College of Human Ecology, Kyung Hee University, Seoul 02447, Korea. jchung@khu.ac.kr
- KMID: 2455837
- DOI: http://doi.org/10.4163/jnh.2018.51.6.489
Abstract
- PURPOSE
Obesity is often associated with disturbances in the mineral metabolism. The purpose of this study was to investigate the effects of high-fat diet-induced obesity on tissue zinc concentrations and zinc transporter expressions in mice.
METHODS
C57BL/6J male mice were fed either a control diet (10% energy from fat, control group) or a high-fat diet (45% energy from fat, obese group) for 15 weeks. The zinc concentrations in the serum, stool, and various tissues were measured by inductively coupled plasma (ICP)-atomic emission spectrophotometry or ICP-mass spectrophotometry. The levels of zinc transporter mRNAs in the liver, duodenum, and pancreas were measured by real-time RT-PCR. The levels of serum adipokines, such as leptin and IL-6, were determined.
RESULTS
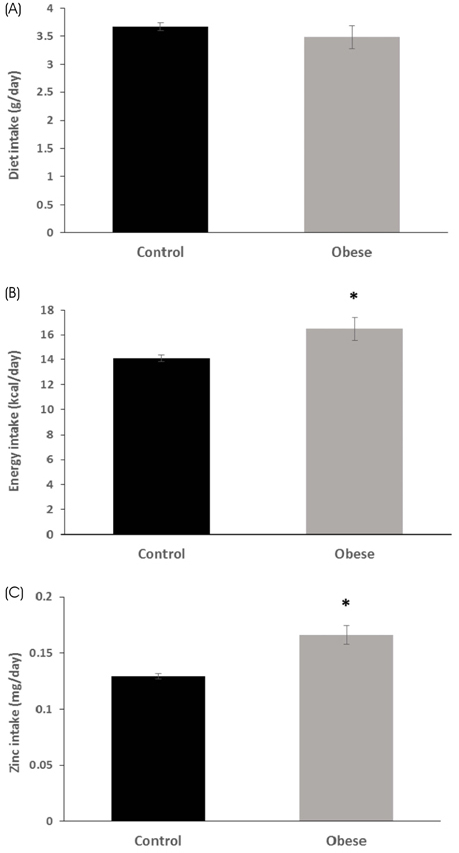
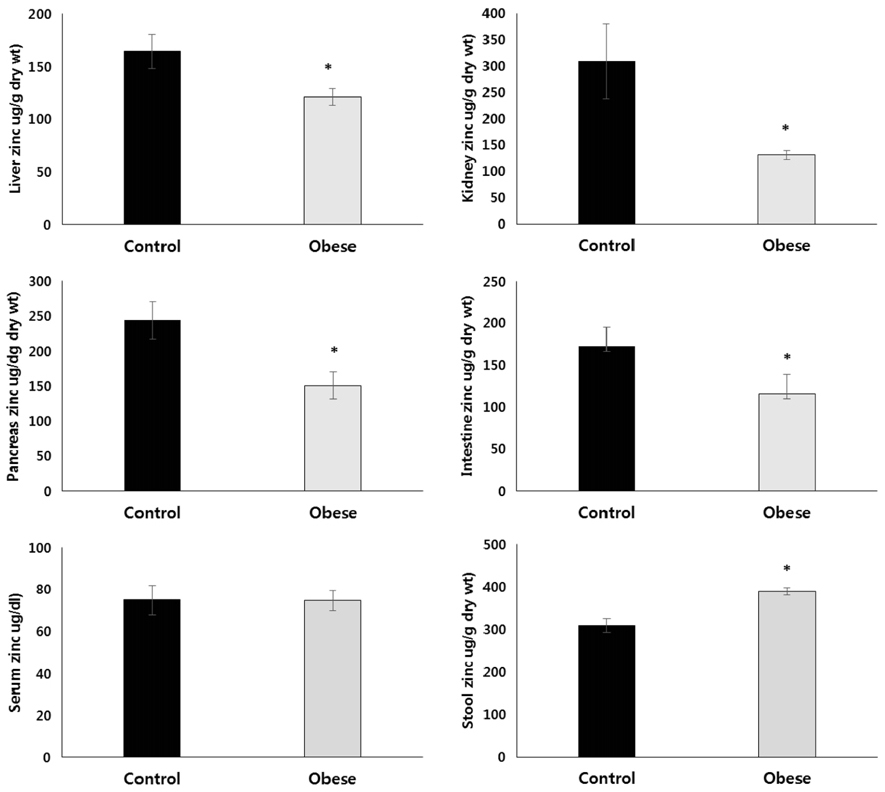
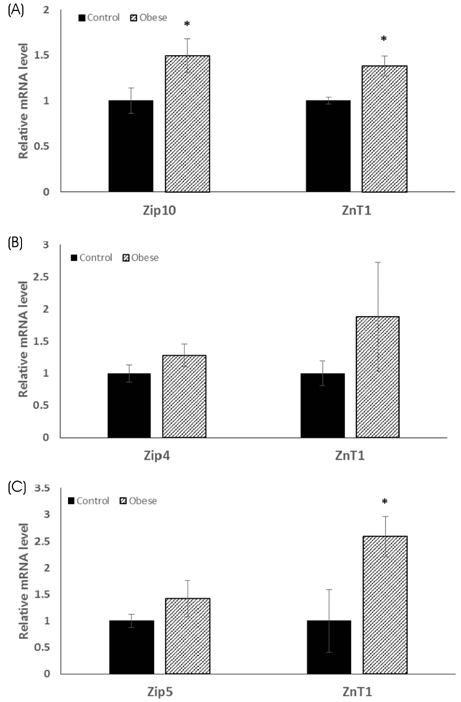
The total body weight, adipose tissue weight, and hepatic TG and cholesterol concentrations were significantly higher in the obese group, as compared to the control group. The obese group had significantly higher levels of serum leptin and pro-inflammatory IL-6 concentrations, and had significantly lower levels of serum alkaline phosphatase activity. The zinc concentrations of the liver, kidney, duodenum, and pancreas were all significantly lower in the obese group than in the control group. On the other hand, the fecal zinc concentrations were significantly higher in the obese group than in the control group. The serum zinc concentrations were not significantly different between the two groups. The ZnT1 mRNA levels of the liver and the pancreas were significantly higher in the obese group, as compared to the control group. Hepatic Zip10 mRNA was also increased in the obese group.
CONCLUSION
Our study findings suggest that obesity increases fecal zinc excretion and lowers the tissue zinc concentrations, which may be associated with alterations in the zinc transporter expressions.
Keyword
MeSH Terms
-
Adipokines
Adipose Tissue
Alkaline Phosphatase
Animals
Body Weight
Cholesterol
Diet
Diet, High-Fat*
Duodenum
Hand
Humans
Interleukin-6
Kidney
Leptin
Liver
Male
Metabolism
Mice*
Miners
Obesity*
Pancreas
Plasma
RNA, Messenger
Spectrophotometry
Zinc*
Adipokines
Alkaline Phosphatase
Cholesterol
Interleukin-6
Leptin
RNA, Messenger
Zinc
Figure
Reference
-
1. Institute of Medicine. Food and Nutrition Board. Panel on Micronutrients. Subcommittees on Upper Reference Levels of Nutrients and of Interpretation and Use of Dietary Reference Intakes. Standing Committee on the Scientific Evaluation of Dietary Reference Intakes. Dietary reference intakes for vitamin A, vitamin K, arsenic, boron, chromium, copper, iodine, iron, manganese, molybdenum, nickel, silicon, vanadium, and zinc. Washington, D.C.: National Academies Press;2001.2. Solomons NW. Mild human zinc deficiency produces an imbalance between cell-mediated and humoral immunity. Nutr Rev. 1998; 56(1 Pt 1):27–28.
Article3. Prasad AS. Zinc: an overview. Nutrition. 1995; 11:1 Suppl. 93–99.4. Heyneman CA. Zinc deficiency and taste disorders. Ann Pharmacother. 1996; 30(2):186–187.
Article5. Wessels I, Maywald M, Rink L. Zinc as a gatekeeper of immune function. Nutrients. 2017; 9(12):E1286.
Article6. Hara T, Takeda TA, Takagishi T, Fukue K, Kambe T, Fukada T. Physiological roles of zinc transporters: molecular and genetic importance in zinc homeostasis. J Physiol Sci. 2017; 67(2):283–301.
Article7. Hojyo S, Fukada T. Zinc transporters and signaling in physiology and pathogenesis. Arch Biochem Biophys. 2016; 611:43–50.
Article8. Lichten LA, Cousins RJ. Mammalian zinc transporters: nutritional and physiologic regulation. Annu Rev Nutr. 2009; 29(1):153–176.
Article9. Dufner-Beattie J, Wang F, Kuo YM, Gitschier J, Eide D, Andrews GK. The acrodermatitis enteropathica gene ZIP4 encodes a tissue-specific, zinc-regulated zinc transporter in mice. J Biol Chem. 2003; 278(35):33474–33481.
Article10. Geiser J, De Lisle RC, Andrews GK. The zinc transporter Zip5 (Slc39a5) regulates intestinal zinc excretion and protects the pancreas against zinc toxicity. PLoS One. 2013; 8(11):e82149.
Article11. Kaler P, Prasad R. Molecular cloning and functional characterization of novel zinc transporter rZip10 (Slc39a10) involved in zinc uptake across rat renal brush-border membrane. Am J Physiol Renal Physiol. 2007; 292(1):F217–F229.
Article12. Cousins RJ, Liuzzi JP, Lichten LA. Mammalian zinc transport, trafficking, and signals. J Biol Chem. 2006; 281(34):24085–24089.
Article13. Ennes Dourado Ferro F, de Sousa Lima VB, Mello Soares NR, Franciscato Cozzolino SM, do Nascimento Marreiro D. Biomarkers of metabolic syndrome and its relationship with the zinc nutritional status in obese women. Nutr Hosp. 2011; 26(3):650–654.14. Konukoglu D, Turhan MS, Ercan M, Serin O. Relationship between plasma leptin and zinc levels and the effect of insulin and oxidative stress on leptin levels in obese diabetic patients. J Nutr Biochem. 2004; 15(12):757–760.
Article15. Marreiro DN, Fisberg M, Cozzolino SM. Zinc nutritional status and its relationships with hyperinsulinemia in obese children and adolescents. Biol Trace Elem Res. 2004; 100(2):137–149.
Article16. Voruganti VS, Cai G, Klohe DM, Jordan KC, Lane MA, Freeland-Graves JH. Short-term weight loss in overweight/obese low-income women improves plasma zinc and metabolic syndrome risk factors. J Trace Elem Med Biol. 2010; 24(4):271–276.
Article17. Ishikawa Y, Kudo H, Kagawa Y, Sakamoto S. Increased plasma levels of zinc in obese adult females on a weight-loss program based on a hypocaloric balanced diet. In Vivo. 2005; 19(6):1035–1037.18. Kennedy ML, Failla ML, Smith JC Jr. Influence of genetic obesity on tissue concentrations of zinc, copper, manganese and iron in mice. J Nutr. 1986; 116(8):1432–1441.
Article19. Chen MD, Lin PY. Zinc-induced hyperleptinemia relates to the amelioration of sucrose-induced obesity with zinc repletion. Obes Res. 2000; 8(7):525–529.
Article20. Francisco V, Pino J, Campos-Cabaleiro V, Ruiz-Fernández C, Mera A, Gonzalez-Gay MA, Gómez R, Gualillo O. Obesity, fat mass and immune system: role for leptin. Front Physiol. 2018; 9:640.
Article21. La Cava A. Leptin in inflammation and autoimmunity. Cytokine. 2017; 98:51–58.
Article22. Sánchez-Margalet V, Martín-Romero C, Santos-Alvarez J, Goberna R, Najib S, Gonzalez-Yanes C. Role of leptin as an immunomodulator of blood mononuclear cells: mechanisms of action. Clin Exp Immunol. 2003; 133(1):11–19.23. Agrawal S, Gollapudi S, Su H, Gupta S. Leptin activates human B cells to secrete TNF-α, IL-6, and IL-10 via JAK2/STAT3 and p38MAPK/ERK1/2 signaling pathway. J Clin Immunol. 2011; 31(3):472–478.
Article24. Zarrati M, Salehi E, Razmpoosh E, Shoormasti RS, Hosseinzadeh-Attar MJ, Shidfar F. Relationship between leptin concentration and body fat with peripheral blood mononuclear cells cytokines among obese and overweight adults. Ir J Med Sci. 2017; 186(1):133–142.
Article25. Besecker BY, Exline MC, Hollyfield J, Phillips G, Disilvestro RA, Wewers MD, Knoell DL. A comparison of zinc metabolism, inflammation, and disease severity in critically ill infected and noninfected adults early after intensive care unit admission. Am J Clin Nutr. 2011; 93(6):1356–1364.
Article26. Alkhouri RH, Hashmi H, Baker RD, Gelfond D, Baker SS. Vitamin and mineral status in patients with inflammatory bowel disease. J Pediatr Gastroenterol Nutr. 2013; 56(1):89–92.
Article27. Griffin IJ, Kim SC, Hicks PD, Liang LK, Abrams SA. Zinc metabolism in adolescents with Crohn’s disease. Pediatr Res. 2004; 56(2):235–239.
Article28. Hennigar SR, Kelley AM, McClung JP. Metallothionein and zinc transporter expression in circulating human blood cells as biomarkers of zinc status: a systematic review. Adv Nutr. 2016; 7(4):735–746.
Article29. Ray CS, Singh B, Jena I, Behera S, Ray S. Low alkaline phosphatase in adult population an indicator of zinc and magnesium deficiency. Curr Res Nutr Food Sci. 2017; 5(3):347–352.30. Cho YE, Lomeda RA, Ryu SH, Sohn HY, Shin HI, Beattie JH, Kwun IS. Zinc deficiency negatively affects alkaline phosphatase and the concentration of Ca, Mg and P in rats. Nutr Res Pract. 2007; 1(2):113–119.
Article31. Krebs NF. Overview of zinc absorption and excretion in the human gastrointestinal tract. J Nutr. 2000; 130:5S Suppl. 1374S–1377S.
Article32. King JC, Shames DM, Woodhouse LR. Zinc homeostasis in humans. J Nutr. 2000; 130:5S Suppl. 1360S–1366S.
Article33. Jackson MJ, Jones DA, Edwards RH, Swainbank IG, Coleman ML. Zinc homeostasis in man: studies using a new stable isotope-dilution technique. Br J Nutr. 1984; 51(2):199–208.
Article34. Tamaki M, Fujitani Y, Hara A, Uchida T, Tamura Y, Takeno K, Kawaguchi M, Watanabe T, Ogihara T, Fukunaka A, Shimizu T, Mita T, Kanazawa A, Imaizumi MO, Abe T, Kiyonari H, Hojyo S, Fukada T, Kawauchi T, Nagamatsu S, Hirano T, Kawamori R, Watada H. The diabetes-susceptible gene SLC30A8/ZnT8 regulates hepatic insulin clearance. J Clin Invest. 2013; 123(10):4513–4524.
Article35. Schmitt S, Küry S, Giraud M, Dréno B, Kharfi M, Bézieau S. An update on mutations of the SLC39A4 gene in acrodermatitis enteropathica. Hum Mutat. 2009; 30(6):926–933.36. Lazarczyk M, Pons C, Mendoza JA, Cassonnet P, Jacob Y, Favre M. Regulation of cellular zinc balance as a potential mechanism of EVER-mediated protection against pathogenesis by cutaneous oncogenic human papillomaviruses. J Exp Med. 2008; 205(1):35–42.
Article37. Lang C, Murgia C, Leong M, Tan LW, Perozzi G, Knight D, Ruffin R, Zalewski P. Anti-inflammatory effects of zinc and alterations in zinc transporter mRNA in mouse models of allergic inflammation. Am J Physiol Lung Cell Mol Physiol. 2007; 292(2):L577–L584.
Article38. Chi ZH, Wang X, Wang ZY, Gao HL, Dahlstrom A, Huang L. Zinc transporter 7 is located in the cis-Golgi apparatus of mouse choroid epithelial cells. Neuroreport. 2006; 17(17):1807–1811.
Article39. Wong CP, Magnusson KR, Ho E. Increased inflammatory response in aged mice is associated with age-related zinc deficiency and zinc transporter dysregulation. J Nutr Biochem. 2013; 24(1):353–359.
Article40. Gálvez-Peralta M, Wang Z, Bao S, Knoell DL, Nebert DW. Tissue-Specific induction of mouse ZIP8 and ZIP14 divalent cation/bicarbonate symporters by, and cytokine response to, inflammatory signals. Int J Toxicol. 2014; 33(3):246–258.
Article41. Miyai T, Hojyo S, Ikawa T, Kawamura M, Irié T, Ogura H, Hijikata A, Bin BH, Yasuda T, Kitamura H, Nakayama M, Ohara O, Yoshida H, Koseki H, Mishima K, Fukada T. Zinc transporter SLC39A10/ZIP10 facilitates antiapoptotic signaling during early B-cell development. Proc Natl Acad Sci U S A. 2014; 111(32):11780–11785.
Article42. Maxel T, Svendsen PF, Smidt K, Lauridsen JK, Brock B, Pedersen SB, Rungby J, Larsen A. Expression patterns and correlations with metabolic markers of zinc transporters ZIP14 and ZNT1 in obesity and polycystic ovary syndrome. Front Endocrinol (Lausanne). 2017; 8:38.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Dietary zinc supplementation in highfat diet-induced obese mice: Effects on the skeletal muscle ZIP7 expression and blood glucose regulation
- The Zinc Transport Systems and Their Regulation in Pathogenic Fungi
- Acrodermatitis Enteropathica in Two Siblings: treated with zinc sulfate
- A Suggestion to Improve Zinc Status of Type 2 Diabetic Women: Relationship among Zn, Protein and Phytate intake
- Concentrations of lead, iron and zinc in blood of coal workers' pneumoconiosis patients