J Periodontal Implant Sci.
2019 Aug;49(4):215-227. 10.5051/jpis.2019.49.4.215.
Periodontal healing using a collagen matrix with periodontal ligament progenitor cells in a dehiscence defect model in beagle dogs
- Affiliations
-
- 1Department of Periodontology, Research Institute of Periodontal Regeneration, Yonsei University College of Dentistry, Seoul, Korea. dentall@yuhs.ac
- 2Department of Applied Life Science, BK21 PLUS Project, Yonsei University College of Dentistry, Seoul, Korea.
- 3Department of Mechanical Engineering, Yonsei University College of Engineering, Seoul, Korea.
- KMID: 2455799
- DOI: http://doi.org/10.5051/jpis.2019.49.4.215
Abstract
- PURPOSE
To histologically characterize periodontal healing at 8 weeks in surgically created dehiscence defects in beagle dogs that received a collagen matrix with periodontal ligament (PDL) progenitor cells.
METHODS
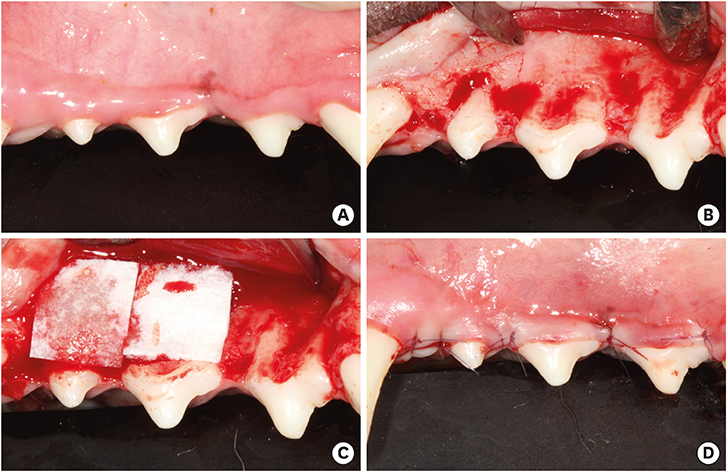
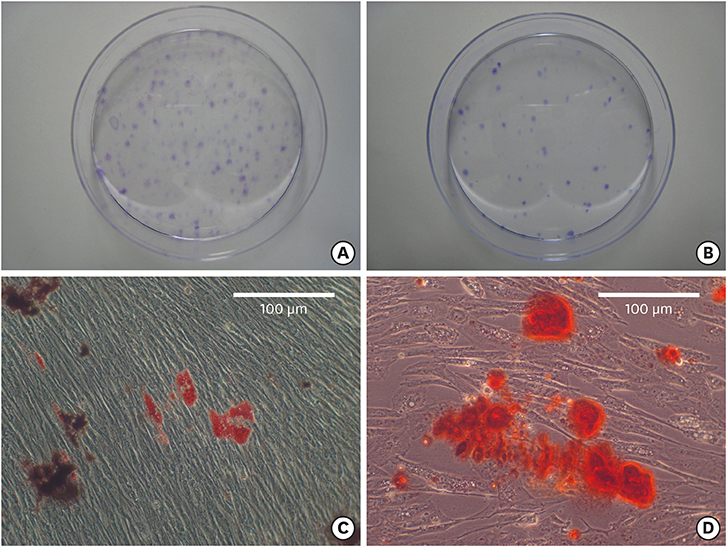
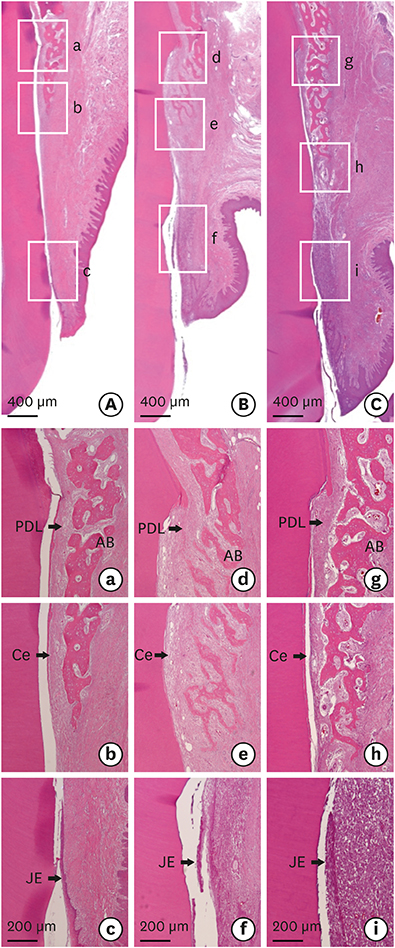
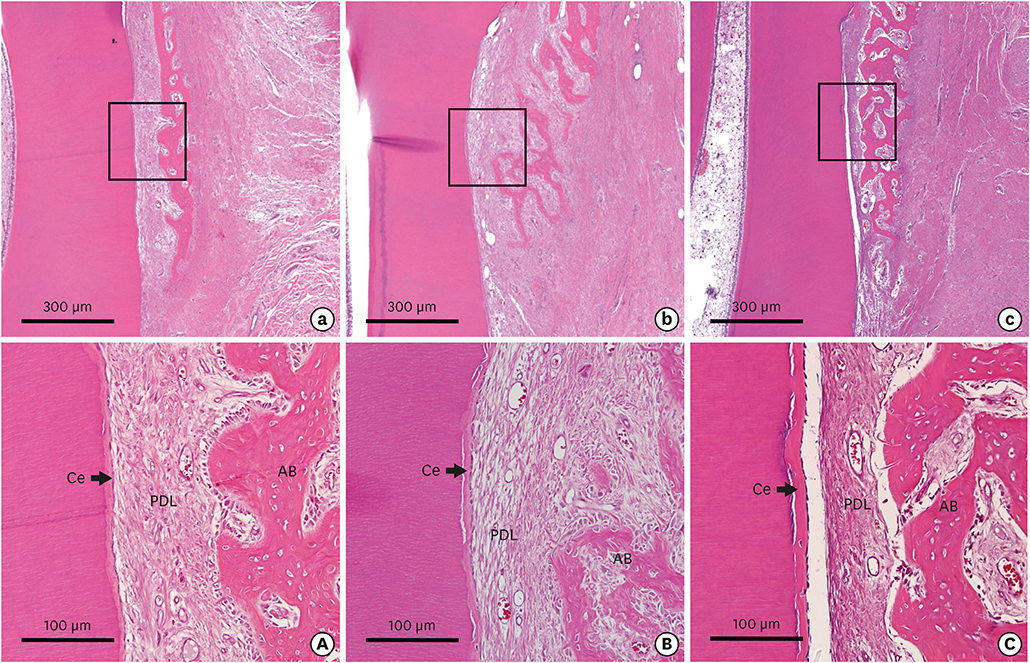
The bilateral maxillary premolars and first molars in 6 animals were used. Standardized experimental dehiscence defects were made on the buccal side of 3 premolars, and primary culturing of PDL progenitor cells was performed on the molars. Collagen matrix was used as a scaffold and a delivery system for PDL progenitor cells. The experimental sites were grafted with collagen matrix (COL), PDL progenitor cells with collagen matrix (COL/CELL), or left without any material (CTL). Histologic and histomorphometric analyses were performed after 8 weeks.
RESULTS
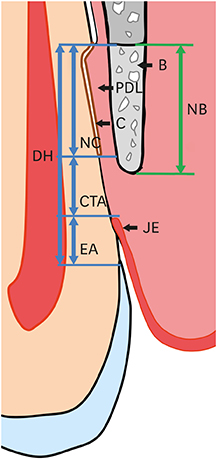
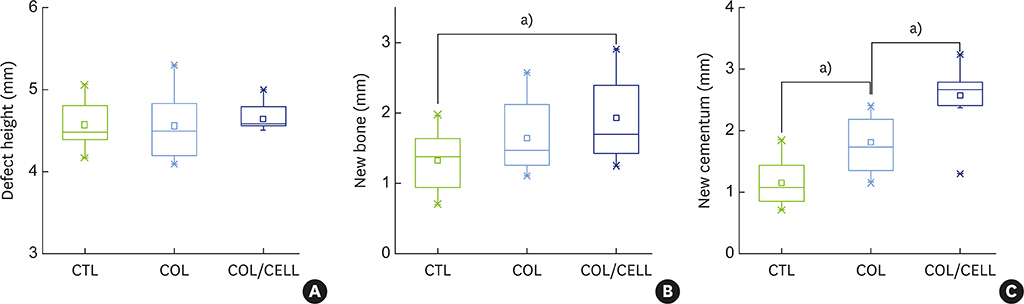
The defect height from the cementoenamel junction to the most apical point of cementum removal did not significantly differ across the CTL, COL, and COL/CELL groups, at 4.57±0.28, 4.56±0.41, and 4.64±0.27 mm (mean ± standard deviation), respectively; the corresponding values for epithelial adhesion were 1.41±0.51, 0.85±0.29, and 0.30±0.41 mm (P<0.05), the heights of new bone regeneration were 1.32±0.44, 1.65±0.52, and 1.93±0.61 mm (P<0.05), and the cementum regeneration values were 1.15±0.42, 1.81±0.46, and 2.57±0.56 mm (P<0.05). There was significantly more new bone formation in the COL/CELL group than in the CTL group, and new cementum length was also significantly higher in the COL/CELL group. However, there were no significant differences in the width of new cementum among the groups.
CONCLUSIONS
PDL progenitor cells carried by a synthetic collagen matrix may enhance periodontal regeneration, including cementum and new bone formation.
Keyword
MeSH Terms
Figure
Reference
-
1. Melcher AH. On the repair potential of periodontal tissues. J Periodontol. 1976; 47:256–260.
Article2. Han J, Menicanin D, Marino V, Ge S, Mrozik K, Gronthos S, et al. Assessment of the regenerative potential of allogeneic periodontal ligament stem cells in a rodent periodontal defect model. J Periodontal Res. 2014; 49:333–345.
Article3. Wikesjö UM, Kean CJ, Zimmerman GJ. Periodontal repair in dogs: supraalveolar defect models for evaluation of safety and efficacy of periodontal reconstructive therapy. J Periodontol. 1994; 65:1151–1157.
Article4. Anitua E, Orive G, Pla R, Roman P, Serrano V, Andía I. The effects of PRGF on bone regeneration and on titanium implant osseointegration in goats: a histologic and histomorphometric study. J Biomed Mater Res A. 2009; 91:158–165.
Article5. Iwasaki K, Komaki M, Yokoyama N, Tanaka Y, Taki A, Kimura Y, et al. Periodontal ligament stem cells possess the characteristics of pericytes. J Periodontol. 2013; 84:1425–1433.
Article6. Kunze M, Huber A, Krajewski A, Lowden E, Schuhmann N, Buening H, et al. Efficient gene transfer to periodontal ligament cells and human gingival fibroblasts by adeno-associated virus vectors. J Dent. 2009; 37:502–508.
Article7. Lin NH, Gronthos S, Bartold PM. Stem cells and future periodontal regeneration. Periodontol 2000. 2009; 51:239–251.
Article8. Liu Y, Zheng Y, Ding G, Fang D, Zhang C, Bartold PM, et al. Periodontal ligament stem cell-mediated treatment for periodontitis in miniature swine. Stem Cells. 2008; 26:1065–1073.
Article9. Thoma DS, Hämmerle CH, Cochran DL, Jones AA, Görlach C, Uebersax L, et al. Soft tissue volume augmentation by the use of collagen-based matrices in the dog mandible -- a histological analysis. J Clin Periodontol. 2011; 38:1063–1070.
Article10. Thoma DS, Villar CC, Cochran DL, Hämmerle CH, Jung RE. Tissue integration of collagen-based matrices: an experimental study in mice. Clin Oral Implants Res. 2012; 23:1333–1339.
Article11. Tsumanuma Y, Iwata T, Washio K, Yoshida T, Yamada A, Takagi R, et al. Comparison of different tissue-derived stem cell sheets for periodontal regeneration in a canine 1-wall defect model. Biomaterials. 2011; 32:5819–5825.
Article12. Lee JS, Kim HS, Park SY, Kim TW, Jung JS, Lee JB, et al. Synergistic effects of a calcium phosphate/fibronectin coating on the adhesion of periodontal ligament stem cells onto decellularized dental root surfaces. Cell Transplant. 2015; 24:1767–1779.
Article13. Bartold PM, Xiao Y, Lyngstaadas SP, Paine ML, Snead ML. Principles and applications of cell delivery systems for periodontal regeneration. Periodontol 2000. 2006; 41:123–135.
Article14. Seo GY, Thoma DS, Jung UW, Lee JS. Increasing the tissue thickness at implant sites using guided bone regeneration and an additional collagen matrix: Histologic observations in beagle dogs. J Biomed Mater Res B Appl Biomater. 2019; 107:741–749.
Article15. Ding G, Liu Y, Wang W, Wei F, Liu D, Fan Z, et al. Allogeneic periodontal ligament stem cell therapy for periodontitis in swine. Stem Cells. 2010; 28:1829–1838.
Article16. Gronthos S, Mrozik K, Shi S, Bartold PM. Ovine periodontal ligament stem cells: isolation, characterization, and differentiation potential. Calcif Tissue Int. 2006; 79:310–317.
Article17. Akizuki T, Oda S, Komaki M, Tsuchioka H, Kawakatsu N, Kikuchi A, et al. Application of periodontal ligament cell sheet for periodontal regeneration: a pilot study in beagle dogs. J Periodontal Res. 2005; 40:245–251.
Article18. Feng F, Akiyama K, Liu Y, Yamaza T, Wang TM, Chen JH, et al. Utility of PDL progenitors for in vivo tissue regeneration: a report of 3 cases. Oral Dis. 2010; 16:20–28.
Article19. Flores MG, Yashiro R, Washio K, Yamato M, Okano T, Ishikawa I. Periodontal ligament cell sheet promotes periodontal regeneration in athymic rats. J Clin Periodontol. 2008; 35:1066–1072.
Article20. Hasegawa M, Yamato M, Kikuchi A, Okano T, Ishikawa I. Human periodontal ligament cell sheets can regenerate periodontal ligament tissue in an athymic rat model. Tissue Eng. 2005; 11:469–478.
Article21. Iwata T, Yamato M, Tsuchioka H, Takagi R, Mukobata S, Washio K, et al. Periodontal regeneration with multi-layered periodontal ligament-derived cell sheets in a canine model. Biomaterials. 2009; 30:2716–2723.
Article22. Kim SH, Kim KH, Seo BM, Koo KT, Kim TI, Seol YJ, et al. Alveolar bone regeneration by transplantation of periodontal ligament stem cells and bone marrow stem cells in a canine peri-implant defect model: a pilot study. J Periodontol. 2009; 80:1815–1823.
Article23. Seo BM, Miura M, Gronthos S, Bartold PM, Batouli S, Brahim J, et al. Investigation of multipotent postnatal stem cells from human periodontal ligament. Lancet. 2004; 364:149–155.
Article24. Mrozik KM, Wada N, Marino V, Richter W, Shi S, Wheeler DL, et al. Regeneration of periodontal tissues using allogeneic periodontal ligament stem cells in an ovine model. Regen Med. 2013; 8:711–723.
Article25. Suaid FF, Ribeiro FV, Gomes TR, Silvério KG, Carvalho MD, Nociti FH Jr, et al. Autologous periodontal ligament cells in the treatment of Class III furcation defects: a study in dogs. J Clin Periodontol. 2012; 39:377–384.
Article26. Volponi AA, Pang Y, Sharpe PT. Stem cell-based biological tooth repair and regeneration. Trends Cell Biol. 2010; 20:715–722.
Article27. Yu N, Oortgiesen DA, Bronckers AL, Yang F, Walboomers XF, Jansen JA. Enhanced periodontal tissue regeneration by periodontal cell implantation. J Clin Periodontol. 2013; 40:698–706.
Article28. Murano Y, Ota M, Katayama A, Sugito H, Shibukawa Y, Yamada S. Periodontal regeneration following transplantation of proliferating tissue derived from periodontal ligament into class III furcation defects in dogs. Biomed Res. 2006; 27:139–147.
Article29. Mathes SH, Wohlwend L, Uebersax L, von Mentlen R, Thoma DS, Jung RE, et al. A bioreactor test system to mimic the biological and mechanical environment of oral soft tissues and to evaluate substitutes for connective tissue grafts. Biotechnol Bioeng. 2010; 107:1029–1039.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- A study on immunocytochemical localization of fibronectin during cytodifferentiation of periodontal ligament fibroblasts in the beagle dogs
- Biological Characteristics of Human Periodontal Ligament Cells
- The Regenerative effects of Platelet-Rich Plasma and Enamel Matrix Protein on Grade III Furcation defects in beagle dogs
- The Effects of DFDB combined with Dura mater on the Periodontal Wound Healing of Dehiscence Defects in Dogs
- The Effects of Graft of DFDB combined with Calcium Sulfate membrane on the Periodontal Wound Healing of Horizontal Dehiscence Defects in Dogs