Korean Circ J.
2019 Sep;49(9):866-876. 10.4070/kcj.2019.0006.
Effect of Ambrisentan Therapy on the Expression of Endothelin Receptor, Endothelial Nitric Oxide Synthase and NADPH Oxidase 4 in Monocrotaline-induced Pulmonary Arterial Hypertension Rat Model
- Affiliations
-
- 1Department of Pediatrics, Ewha Womans University College of Medicine, Seoul, Korea. ymhong@ewha.ac.kr
- 2Department of Thoracic and Cardiovascular Surgery, Ewha Womans University College of Medicine, Seoul, Korea.
- KMID: 2455794
- DOI: http://doi.org/10.4070/kcj.2019.0006
Abstract
- BACKGROUND AND OBJECTIVES
Elevated endothelin (ET)-1 level is strongly correlated with the pathogenesis of pulmonary arterial hypertension (PAH). Expression level of nicotinamide adenine dinucleotide phosphate oxidase (NOX) 4 is increased in the PAH patients. Ambrisentan, a selective endothelin receptor A (ERA) antagonist, is widely used in PAH therapy. The current study was undertaken to evaluate the effects of ambrisentan treatment in the monocrotaline (MCT)-induced PAH rat model.
METHODS
Rats were categorized into control group (C), monocrotaline group (M) and ambrisentan group (Am). The M and Am were subcutaneously injected 60 mg/kg MCT at day 0, and in Am, ambrisentan was orally administered the day after MCT injection for 4 weeks. The right ventricle (RV) pressure was measured and pathological changes of the lung tissues were observed by Victoria blue staining. Protein expressions of ET-1, ERA, endothelial nitric oxide synthase (eNOS) and NOX4 were confirmed by western blot analysis.
RESULTS
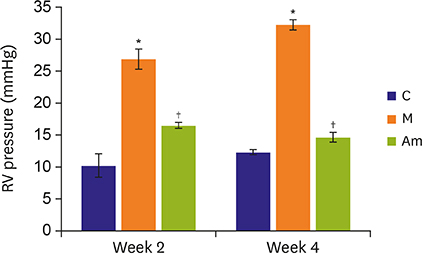
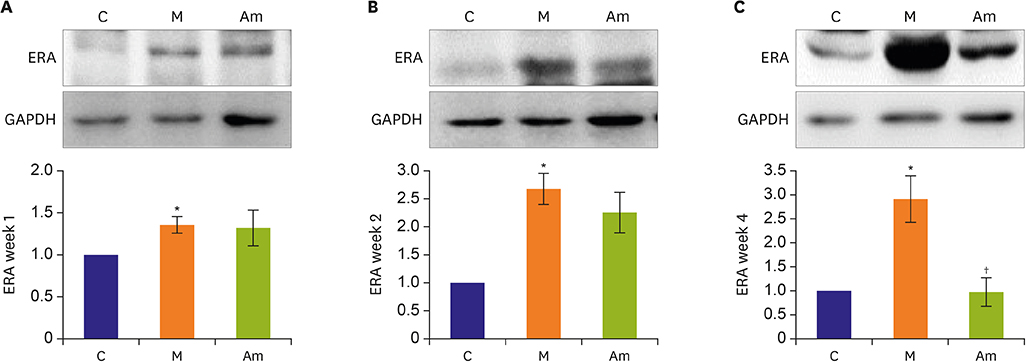
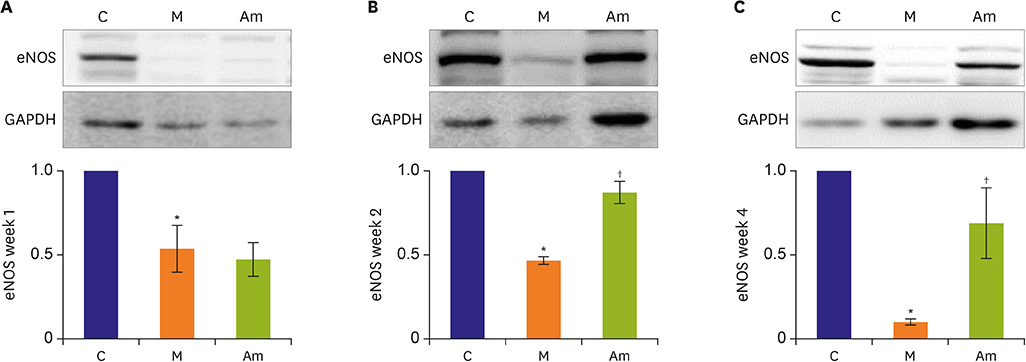
Ambrisentan treatment resulted in a recovery of the body weight and RV/left ventricle+septum at week 4. The RV pressure was lowered at weeks 2 and 4 after ambrisentan administration. Medial wall thickening of pulmonary arterioles and the number of intra-acinar arteries were also attenuated by ambrisentan at week 4. Protein expression levels of ET-1 and eNOS were recovered at weeks 2 and 4, and ERA levels recovered at week 4.
CONCLUSIONS
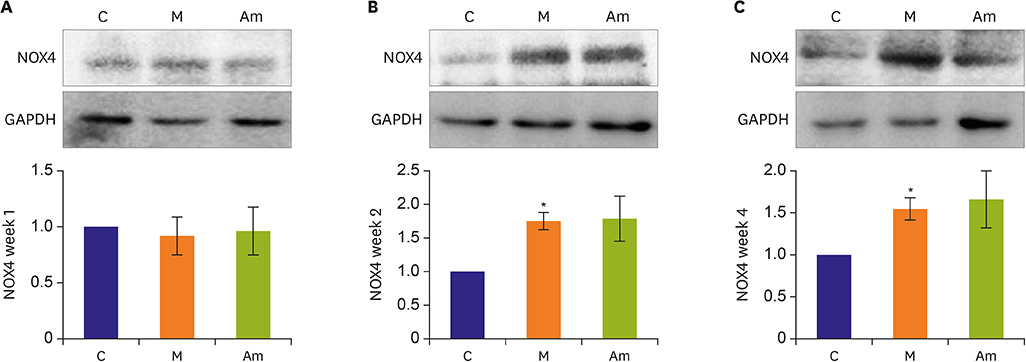
Ambrisentan administration resulted in the recovery of ET-1, ERA and eNOS protein expression levels in the PAH model. However, the expression level of NOX4 remained unaffected after ambrisentan treatment.
MeSH Terms
-
Animals
Arteries
Arterioles
Blotting, Western
Body Weight
Endothelin Receptor Antagonists
Endothelins*
Gene Expression
Heart Ventricles
Humans
Hypertension*
Hypertension, Pulmonary
Lung
Models, Animal*
Monocrotaline
NADP*
NADPH Oxidase*
Nitric Oxide Synthase Type III*
Oxidoreductases
Rats*
Receptors, Endothelin*
Victoria
Endothelin Receptor Antagonists
Endothelins
Monocrotaline
NADP
NADPH Oxidase
Nitric Oxide Synthase Type III
Oxidoreductases
Receptors, Endothelin
Figure
Cited by 2 articles
-
Moving Beyond the Endothelium is Still Challenging-Complex Interplay between Endothelin and Reactive Oxygen Species in Pulmonary Arterial Hypertension
Hyemoon Chung, Il Suk Sohn
Korean Circ J. 2019;49(9):877-878. doi: 10.4070/kcj.2019.0167.Stem Cell and Exosome Therapy in Pulmonary Hypertension
Seyeon Oh, Ji-Hye Jung, Kyung-Jin Ahn, Albert Youngwoo Jang, Kyunghee Byun, Phillip C. Yang, Wook-Jin Chung
Korean Circ J. 2022;52(2):110-122. doi: 10.4070/kcj.2021.0191.
Reference
-
1. Patel BB, Feng Y, Cheng-Lai A. Pulmonary arterial hypertension: a review in pharmacotherapy. Cardiol Rev. 2015; 23:33–51.2. Tuder RM, Abman SH, Braun T, et al. Development and pathology of pulmonary hypertension. J Am Coll Cardiol. 2009; 54:S3–S9.
Article3. Cooke JP. A novel mechanism for pulmonary arterial hypertension? Circulation. 2003; 108:1420–1421.
Article4. Chester AH, Yacoub MH. The role of endothelin-1 in pulmonary arterial hypertension. Glob Cardiol Sci Pract. 2014; 2014:62–78.
Article5. Giaid A, Yanagisawa M, Langleben D, et al. Expression of endothelin-1 in the lungs of patients with pulmonary hypertension. N Engl J Med. 1993; 328:1732–1739.
Article6. Jernigan NL, Walker BR, Resta TC. Endothelium-derived reactive oxygen species and endothelin-1 attenuate NO-dependent pulmonary vasodilation following chronic hypoxia. Am J Physiol Lung Cell Mol Physiol. 2004; 287:L801–L808.
Article7. Ismail S, Sturrock A, Wu P, et al. NOX4 mediates hypoxia-induced proliferation of human pulmonary artery smooth muscle cells: the role of autocrine production of transforming growth factor-beta1 and insulin-like growth factor binding protein-3. Am J Physiol Lung Cell Mol Physiol. 2009; 296:L489–L499.8. Craige SM, Chen K, Pei Y, et al. NADPH oxidase 4 promotes endothelial angiogenesis through endothelial nitric oxide synthase activation. Circulation. 2011; 124:731–740.
Article9. Lee H, Kim KC, Hong YM. Change of voltage-gated potassium channel 1.7 expressions in monocrotaline-induced pulmonary arterial hypertension rat model. Korean J Pediatr. 2018; 61:271–278.
Article10. Li L, Fink GD, Watts SW, et al. Endothelin-1 increases vascular superoxide via endothelin(A)-NADPH oxidase pathway in low-renin hypertension. Circulation. 2003; 107:1053–1058.11. Dong F, Zhang X, Wold LE, Ren Q, Zhang Z, Ren J. Endothelin-1 enhances oxidative stress, cell proliferation and reduces apoptosis in human umbilical vein endothelial cells: role of ETB receptor, NADPH oxidase and caveolin-1. Br J Pharmacol. 2005; 145:323–333.
Article12. Bowers R, Cool C, Murphy RC, et al. Oxidative stress in severe pulmonary hypertension. Am J Respir Crit Care Med. 2004; 169:764–769.
Article13. Casserly B, Klinger JR. Ambrisentan for the treatment of pulmonary arterial hypertension. Drug Des Devel Ther. 2009; 2:265–280.14. Kim KC, Lee JC, Lee H, Cho MS, Choi SJ, Hong YM. Changes in Caspase-3, B cell leukemia/lymphoma-2, interleukin-6, tumor necrosis factor-α and vascular endothelial growth factor gene expression after human umbilical cord blood derived mesenchymal stem cells transfusion in pulmonary hypertension rat models. Korean Circ J. 2016; 46:79–92.
Article15. Lee H, Kim KC, Cho MS, Suh SH, Hong YM. Modafinil improves monocrotaline-induced pulmonary hypertension rat model. Pediatr Res. 2016; 80:119–127.
Article16. Lim KA, Kim KC, Cho MS, Lee BE, Kim HS, Hong YM. Gene expression of endothelin-1 and endothelin receptor a on monocrotaline-induced pulmonary hypertension in rats after bosentan treatment. Korean Circ J. 2010; 40:459–464.
Article17. Sun LR, Wang C, Wu AQ, et al. Gene polymorphism of the endothelial nitric oxide synthase enzyme and pulmonary hypertension in patient with chronic obstructive pulmonary disease. Zhonghua Jie He He Hu Xi Za Zhi. 2008; 31:335–340.18. Giaid A, Saleh D. Reduced expression of endothelial nitric oxide synthase in the lungs of patients with pulmonary hypertension. N Engl J Med. 1995; 333:214–221.
Article19. Wu F, Hao Y, Yang J, et al. Protective effects of aloperine on monocrotaline-induced pulmonary hypertension in rats. Biomed Pharmacother. 2017; 89:632–641.
Article20. Sturrock A, Cahill B, Norman K, et al. Transforming growth factor-beta1 induces Nox4 NAD(P)H oxidase and reactive oxygen species-dependent proliferation in human pulmonary artery smooth muscle cells. Am J Physiol Lung Cell Mol Physiol. 2006; 290:L661–73.21. Barman SA, Fulton D. Adventitial fibroblast Nox4 expression and ROS signaling in pulmonary arterial hypertension. Adv Exp Med Biol. 2017; 967:1–11.
Article22. Peng JJ, Liu B, Xu JY, Peng J, Luo XJ. NADPH oxidase: its potential role in promotion of pulmonary arterial hypertension. Naunyn Schmiedebergs Arch Pharmacol. 2017; 390:331–338.
Article23. Koo HS, Kim KC, Hong YM. Gene expressions of nitric oxide synthase and matrix metalloproteinase-2 in monocrotaline-induced pulmonary hypertension in rats after bosentan treatment. Korean Circ J. 2011; 41:83–90.
Article24. Kosanovic D, Kojonazarov B, Luitel H, et al. Therapeutic efficacy of TBC3711 in monocrotaline-induced pulmonary hypertension. Respir Res. 2011; 12:87.
Article25. Jasmin JF, Cernacek P, Dupuis J. Activation of the right ventricular endothelin (ET) system in the monocrotaline model of pulmonary hypertension: response to chronic ETA receptor blockade. Clin Sci (Lond). 2003; 105:647–653.
Article26. Huang PL, Huang Z, Mashimo H, et al. Hypertension in mice lacking the gene for endothelial nitric oxide synthase. Nature. 1995; 377:239–242.
Article27. Dubois M, Delannoy E, Duluc L, et al. Biopterin metabolism and eNOS expression during hypoxic pulmonary hypertension in mice. PLoS One. 2013; 8:e82594.
Article28. Kingman M, Ruggiero R, Torres F. Ambrisentan, an endothelin receptor type A-selective endothelin receptor antagonist, for the treatment of pulmonary arterial hypertension. Expert Opin Pharmacother. 2009; 10:1847–1858.
Article29. Barst RJ. A review of pulmonary arterial hypertension: role of ambrisentan. Vasc Health Risk Manag. 2007; 3:11–22.30. Iglarz M, Steiner P, Wanner D, Rey M, Hess P, Clozel M. Vascular effects of endothelin receptor antagonists depends on their selectivity for ETA versus ETB receptors and on the functionality of endothelial ETB receptors. J Cardiovasc Pharmacol. 2015; 66:332–337.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- An inhibitory effect of tumor necrosis factor-alpha antagonist to gene expression in monocrotaline-induced pulmonary hypertensive rats model
- Changes of Pulmonary Pathology and Gene Expressions After Simvastatin Treatment in the Monocrotaline-Induced Pulmonary Hypertension Rat Model
- The Expression of NADPH-diaphorase in Corneal Neovascularization of streptozotocin Induced Diabetic Rat
- Gene Expressions of Nitric Oxide Synthase and Matrix Metalloproteinase-2 in Monocrotaline-Induced Pulmonary Hypertension in Rats After Bosentan Treatment
- Resveratrol Attenuates Monocrotaline-Induced Pulmonary Hypertension in Rats