Diabetes Metab J.
2019 Aug;43(4):474-486. 10.4093/dmj.2018.0171.
Plasma Fetuin-A Levels and Risk of Type 2 Diabetes Mellitus in A Chinese Population: A Nested Case-Control Study
- Affiliations
-
- 1Health Services and Systems Research, Duke-NUS Medical School, Singapore. woonpuay.koh@duke-nus.edu.sg
- 2Saw Swee Hock School of Public Health, National University of Singapore and National University Health System, Singapore.
- 3Department of Nutrition, Harvard T.H. Chan School of Public Health, Boston, MA, USA.
- 4Channing Division of Network Medicine, Department of Medicine, Brigham and Women's Hospital, Harvard Medical School, Boston, MA, USA.
- 5Division of Cancer Control and Population Sciences, UPMC Hillman Cancer Center, University of Pittsburgh, Pittsburgh, PA, USA.
- 6Department of Epidemiology, Graduate School of Public Health, University of Pittsburgh, Pittsburgh, PA, USA.
- 7Department of Epidemiology and Biostatistics, School of Public Health, Tongji Medical College, Huazhong University of Science and Technology, Wuhan, China. panan@hust.edu.cn
- KMID: 2455670
- DOI: http://doi.org/10.4093/dmj.2018.0171
Abstract
- BACKGROUND
Fetuin-A is a hepatokine that involved in the pathogenesis of insulin resistance. Previous epidemiological studies have found a positive association between blood fetuin-A and type 2 diabetes mellitus (T2DM) risk among Caucasians and African Americans. We aimed to investigate the prospective relationship between fetuin-A and T2DM in an Asian population for the first time.
METHODS
A nested case-control study was established within a prospective cohort of Chinese living in Singapore. At blood collection (1999 to 2004), all participants were free of diagnosed T2DM and aged 50 to 79 years. At subsequent follow-up (2006 to 2010), 558 people reported to have T2DM and were classified as incident cases, and 558 controls were randomly chosen from the participants who did not develop T2DM to match with cases on age, sex, dialect group, and date of blood collection. Plasma fetuin-A levels were measured retrospectively in cases and controls using samples collected at baseline. Conditional logistic regression models were used to compute the odds ratio (OR) and 95% confidence interval (CI). Restricted cubic spline analysis was used to examine a potential non-linear association between fetuin-A levels and T2DM risk.
RESULTS
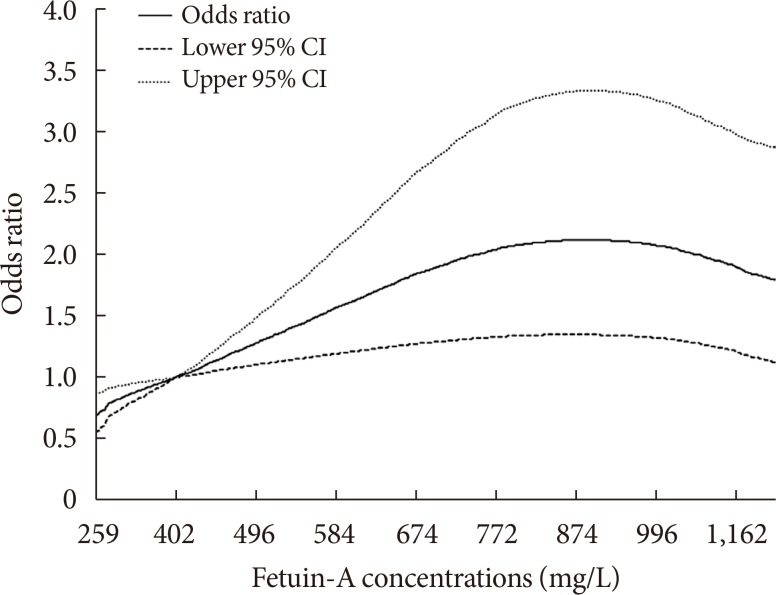
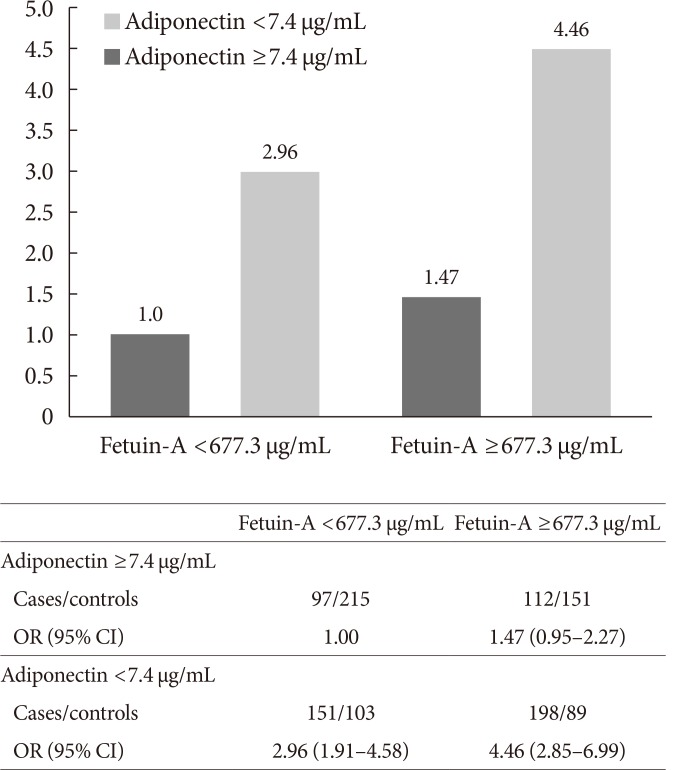
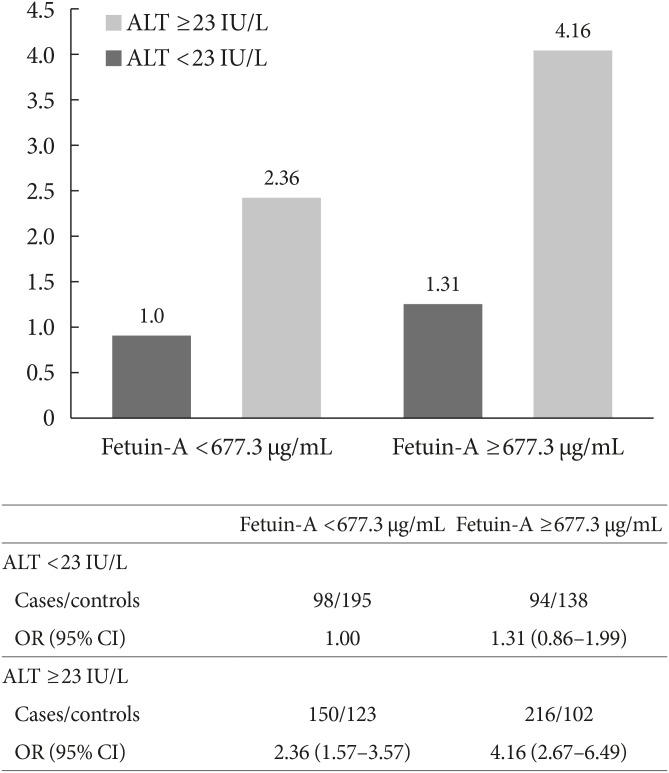
Compared with those in the lowest fetuin-A quintile, participants in the highest quintile had a two-fold increased risk of developing T2DM (OR, 2.06; 95% CI, 1.21 to 3.51). A non-linear association was observed (P nonlinearity=0.005), where the association between fetuin-A levels and T2DM risk plateaued at plasma concentrations around 830 µg/mL.
CONCLUSION
There is a positive association between plasma fetuin-A levels and risk of developing T2DM in this Chinese population.
Keyword
MeSH Terms
-
African Americans
alpha-2-HS-Glycoprotein*
Asian Continental Ancestry Group*
Case-Control Studies*
Cohort Studies
Diabetes Mellitus, Type 2*
Epidemiologic Studies
Epidemiology
Follow-Up Studies
Humans
Insulin Resistance
Logistic Models
Odds Ratio
Plasma*
Prospective Studies
Retrospective Studies
Singapore
alpha-2-HS-Glycoprotein
Figure
Reference
-
1. Levinthal GN, Tavill AS. Liver disease and diabetes mellitus. Clin Diabetes. 1999; 17:1–20.2. Rauth G, Poschke O, Fink E, Eulitz M, Tippmer S, Kellerer M, Haring HU, Nawratil P, Haasemann M, Jahnen-Dechent W, Muller-Esterl W. The nucleotide and partial amino acid sequences of rat fetuin. Identity with the natural tyrosine kinase inhibitor of the rat insulin receptor. Eur J Biochem. 1992; 204:523–529. PMID: 1371750.
Article3. Auberger P, Falquerho L, Contreres JO, Pages G, Le Cam G, Rossi B, Le Cam A. Characterization of a natural inhibitor of the insulin receptor tyrosine kinase: cDNA cloning, purification, and anti-mitogenic activity. Cell. 1989; 58:631–640. PMID: 2766355.
Article4. Mathews ST, Singh GP, Ranalletta M, Cintron VJ, Qiang X, Goustin AS, Jen KL, Charron MJ, Jahnen-Dechent W, Grunberger G. Improved insulin sensitivity and resistance to weight gain in mice null for the Ahsg gene. Diabetes. 2002; 51:2450–2458. PMID: 12145157.
Article5. Hennige AM, Staiger H, Wicke C, Machicao F, Fritsche A, Haring HU, Stefan N. Fetuin-A induces cytokine expression and suppresses adiponectin production. PLoS One. 2008; 3:e1765. PMID: 18335040.
Article6. Guo VY, Cao B, Cai C, Cheng KK, Cheung BMY. Fetuin-A levels and risk of type 2 diabetes mellitus: a systematic review and meta-analysis. Acta Diabetol. 2018; 55:87–98. PMID: 29127490.
Article7. Ix JH, Wassel CL, Kanaya AM, Vittinghoff E, Johnson KC, Koster A, Cauley JA, Harris TB, Cummings SR, Shlipak MG;. Fetuin-A and incident diabetes mellitus in older persons. JAMA. 2008; 300:182–188. PMID: 18612115.
Article8. Stefan N, Fritsche A, Weikert C, Boeing H, Joost HG, Haring HU, Schulze MB. Plasma fetuin-A levels and the risk of type 2 diabetes. Diabetes. 2008; 57:2762–2767. PMID: 18633113.
Article9. Ix JH, Biggs ML, Mukamal KJ, Kizer JR, Zieman SJ, Siscovick DS, Mozzaffarian D, Jensen MK, Nelson L, Ruderman N, Djousse L. Association of fetuin-A with incident diabetes mellitus in community-living older adults: the cardiovascular health study. Circulation. 2012; 125:2316–2322. PMID: 22511752.10. Laughlin GA, Barrett-Connor E, Cummins KM, Daniels LB, Wassel CL, Ix JH. Sex-specific association of fetuin-A with type 2 diabetes in older community-dwelling adults: the Rancho Bernardo study. Diabetes Care. 2013; 36:1994–2000. PMID: 23315604.
Article11. Sun Q, Cornelis MC, Manson JE, Hu FB. Plasma levels of fetuin-A and hepatic enzymes and risk of type 2 diabetes in women in the U.S. Diabetes. 2013; 62:49–55. PMID: 22923470.
Article12. Aroner SA, Mukamal KJ, St-Jules DE, Budoff MJ, Katz R, Criqui MH, Allison MA, de Boer IH, Siscovick DS, Ix JH, Jensen MK. Fetuin-a and risk of diabetes independent of liver fat content: the multi-ethnic study of atherosclerosis. Am J Epidemiol. 2017; 185:54–64. PMID: 27856445.13. Dutta D, Mondal SA, Kumar M, Hasanoor Reza AH, Biswas D, Singh P, Chakrabarti S, Mukhopadhyay S. Serum fetuin-A concentration predicts glycaemic outcomes in people with prediabetes: a prospective study from eastern India. Diabet Med. 2014; 31:1594–1599. PMID: 24975463.
Article14. Ma RC, Chan JC. Type 2 diabetes in East Asians: similarities and differences with populations in Europe and the United States. Ann N Y Acad Sci. 2013; 1281:64–91. PMID: 23551121.
Article15. Doi Y, Kubo M, Yonemoto K, Ninomiya T, Iwase M, Tanizaki Y, Shikata K, Iida M, Kiyohara Y. Liver enzymes as a predictor for incident diabetes in a Japanese population: the Hisayama study. Obesity (Silver Spring). 2007; 15:1841–1850. PMID: 17636103.
Article16. Wang YL, Koh WP, Yuan JM, Pan A. Association between liver enzymes and incident type 2 diabetes in Singapore Chinese men and women. BMJ Open Diabetes Res Care. 2016; 4:e000296.
Article17. Lorenzo C, Hanley AJ, Rewers MJ, Haffner SM. Discriminatory value of alanine aminotransferase for diabetes prediction: the Insulin Resistance Atherosclerosis Study. Diabet Med. 2016; 33:348–355. PMID: 26094705.
Article18. Kunutsor SK, Apekey TA, Walley J. Liver aminotransferases and risk of incident type 2 diabetes: a systematic review and meta-analysis. Am J Epidemiol. 2013; 178:159–171. PMID: 23729682.
Article19. Chan JC, Yeung R, Luk A. The Asian diabetes phenotypes: challenges and opportunities. Diabetes Res Clin Pract. 2014; 105:135–139. PMID: 24947419.
Article20. Hankin JH, Stram DO, Arakawa K, Park S, Low SH, Lee HP, Yu MC. Singapore Chinese Health Study: development, validation, and calibration of the quantitative food frequency questionnaire. Nutr Cancer. 2001; 39:187–195. PMID: 11759279.
Article21. Odegaard AO, Pereira MA, Koh WP, Arakawa K, Lee HP, Yu MC. Coffee, tea, and incident type 2 diabetes: the Singapore Chinese Health Study. Am J Clin Nutr. 2008; 88:979–985. PMID: 18842784.
Article22. Roshanzamir F, Miraghajani M, Rouhani MH, Mansourian M, Ghiasvand R, Safavi SM. The association between circulating fetuin-A levels and type 2 diabetes mellitus risk: systematic review and meta-analysis of observational studies. J Endocrinol Invest. 2018; 41:33–47. PMID: 28643299.
Article23. Reinehr T, Roth CL. Fetuin-A and its relation to metabolic syndrome and fatty liver disease in obese children before and after weight loss. J Clin Endocrinol Metab. 2008; 93:4479–4485. PMID: 18728159.
Article24. Siddiq A, Lepretre F, Hercberg S, Froguel P, Gibson F. A synonymous coding polymorphism in the alpha2-Heremans-schmid glycoprotein gene is associated with type 2 diabetes in French Caucasians. Diabetes. 2005; 54:2477–2481. PMID: 16046317.25. Ix JH, Sharma K. Mechanisms linking obesity, chronic kidney disease, and fatty liver disease: the roles of fetuin-A, adiponectin, and AMPK. J Am Soc Nephrol. 2010; 21:406–412. PMID: 20150538.
Article26. Li S, Shin HJ, Ding EL, van Dam RM. Adiponectin levels and risk of type 2 diabetes: a systematic review and meta-analysis. JAMA. 2009; 302:179–188. PMID: 19584347.
Article27. Ix JH, Shlipak MG, Brandenburg VM, Ali S, Ketteler M, Whooley MA. Association between human fetuin-A and the metabolic syndrome: data from the Heart and Soul Study. Circulation. 2006; 113:1760–1767. PMID: 16567568.28. Dasgupta S, Bhattacharya S, Biswas A, Majumdar SS, Mukhopadhyay S, Ray S, Bhattacharya S. NF-kappaB mediates lipid-induced fetuin-A expression in hepatocytes that impairs adipocyte function effecting insulin resistance. Biochem J. 2010; 429:451–462. PMID: 20482516.29. Stefan N, Hennige AM, Staiger H, Machann J, Schick F, Krober SM, Machicao F, Fritsche A, Haring HU. Alpha2-Heremans-Schmid glycoprotein/fetuin-A is associated with insulin resistance and fat accumulation in the liver in humans. Diabetes Care. 2006; 29:853–857. PMID: 16567827.30. Stefan N, Schick F, Haring HU. Ectopic fat in insulin resistance, dyslipidemia, and cardiometabolic disease. N Engl J Med. 2014; 371:2236–2237.
Article31. Stefan N, Sun Q, Fritsche A, Machann J, Schick F, Gerst F, Jeppesen C, Joost HG, Hu FB, Boeing H, Ullrich S, Haring HU, Schulze MB. Impact of the adipokine adiponectin and the hepatokine fetuin-A on the development of type 2 diabetes: prospective cohort- and cross-sectional phenotyping studies. PLoS One. 2014; 9:e92238. PMID: 24643166.
Article32. Kotsopoulos J, Tworoger SS, Campos H, Chung FL, Clevenger CV, Franke AA, Mantzoros CS, Ricchiuti V, Willett WC, Hankinson SE, Eliassen AH. Reproducibility of plasma and urine biomarkers among premenopausal and postmenopausal women from the Nurses' Health Studies. Cancer Epidemiol Biomarkers Prev. 2010; 19:938–946. PMID: 20332276.33. Stefan N, Haring HU. Circulating fetuin-A and free fatty acids interact to predict insulin resistance in humans. Nat Med. 2013; 19:394–395. PMID: 23558619.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Plasma Paraoxonase Activities in Type 2 Diabetes Mellitus
- Serum Visfatin and Fetuin-A Levels and Glycemic Control in Patients with Obese Type 2 Diabetes Mellitus
- Homocysteine as a Risk Factor for Development of Microalbuminuria in Type 2 Diabetes
- Multiple Biomarkers Improved Prediction for the Risk of Type 2 Diabetes Mellitus in Singapore Chinese Men and Women
- The Role of Plasma Adiponectin and Polymorphism of Adiponectin Gene in the Development of Type 2 Diabetes Mellitus