Diabetes Metab J.
2019 Aug;43(4):432-446. 10.4093/dmj.2018.0092.
Effectiveness and Safety of Adding Basal Insulin Glargine in Patients with Type 2 Diabetes Mellitus Exhibiting Inadequate Response to Metformin and DPP-4 Inhibitors with or without Sulfonylurea
- Affiliations
-
- 1Department of Internal Medicine, Asan Medical Center, University of Ulsan College of Medicine, Seoul, Korea. jypark@amc.seoul.kr
- 2Division of Endocrinology and Metabolism, Department of Internal Medicine, Seoul St. Mary's Hospital, College of Medicine, The Catholic University of Korea, Seoul, Korea.
- 3Department of Internal Medicine, Kangwon National University School of Medicine, Chuncheon, Korea.
- 4Department of Internal Medicine, Konkuk University Medical Center, Konkuk University School of Medicine, Seoul, Korea.
- 5Division of Endocrinology and Metabolism, Department of Internal Medicine, Korea University College of Medicine, Seoul, Korea.
- 6Division of Endocrinology and Metabolism, Department of Medicine, Thyroid Center, Samsung Medical Center, Sungkyunkwan University School of Medicine, Seoul, Korea.
- 7Department of Internal Medicine, Seoul National University Hospital, Seoul National University College of Medicine, Seoul, Korea.
- 8Division of Endocrinology and Metabolism, Department of Internal Medicine, Chonbuk National University Hospital, Chonbuk National University Medical School, Jeonju, Korea.
- 9Department of Internal Medicine, Chungnam National University College of Medicine, Daejeon, Korea.
- 10Department of Internal Medicine, Jeju National University School of Medicine, Jeju, Korea.
- 11Medical Department of Diabetes and Cardiovascular, Sanofi-Korea, Seoul, Korea.
- 12Division of Endocrinology and Metabolism, Department of Internal Medicine, Graduate School, Yonsei University College of Medicine, Seoul, Korea. bwanlee@yuhs.ac.kr
- KMID: 2455667
- DOI: http://doi.org/10.4093/dmj.2018.0092
Abstract
- BACKGROUND
We aimed to investigate the effectiveness and safety of adding basal insulin to initiating dipeptidyl peptidase-4 (DPP-4) inhibitor and metformin and/or sulfonylurea (SU) in achieving the target glycosylated hemoglobin (HbA1c) in patients with type 2 diabetes mellitus (T2DM).
METHODS
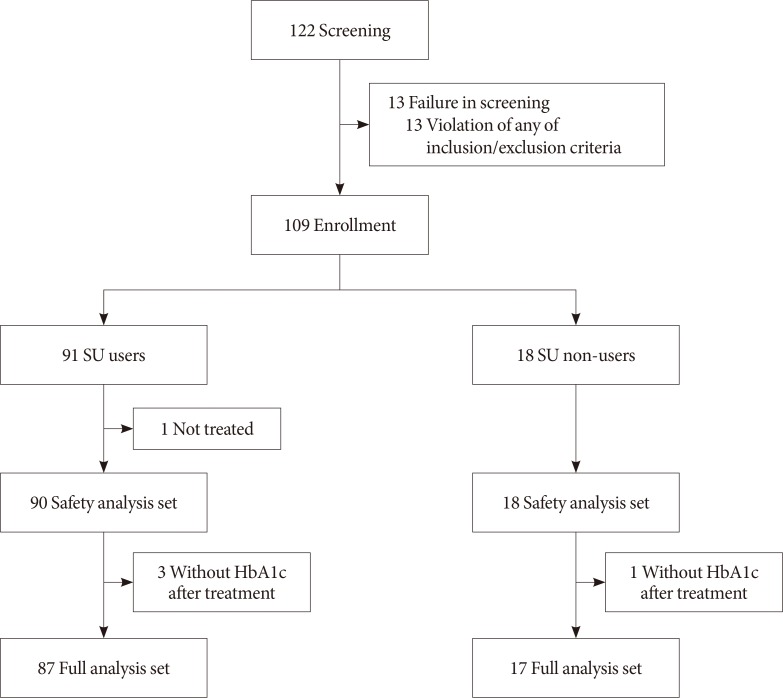
This was a single-arm, multicenter, 24-week, open-label, phase 4 study in patients with inadequately controlled (HbA1c ≥7.5%) T2DM despite the use of DPP-4 inhibitor and metformin. A total of 108 patients received insulin glargine while continuing oral antidiabetic drugs (OADs). The primary efficacy endpoint was the percentage of subjects achieving HbA1c ≤7.0%. Other glycemic profiles were also evaluated, and the safety endpoints were adverse events (AEs) and hypoglycemia.
RESULTS
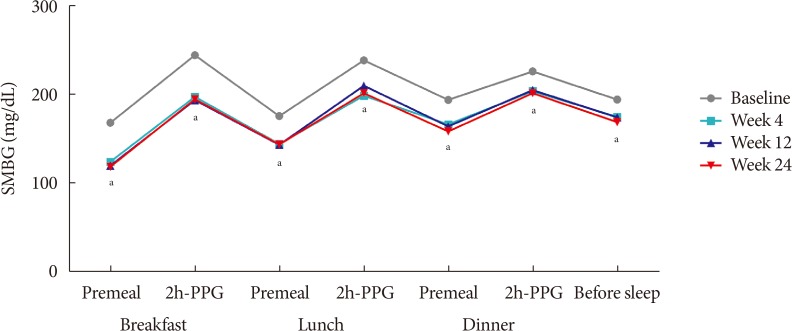
The median HbA1c at baseline (8.9%; range, 7.5% to 11.1%) decreased to 7.6% (5.5% to 11.7%) at 24 weeks. Overall, 31.7% subjects (n=33) achieved the target HbA1c level of ≤7.0%. The mean differences in body weight and fasting plasma glucose were 1.2±3.4 kg and 56.0±49.8 mg/dL, respectively. Hypoglycemia was reported in 36 subjects (33.3%, 112 episodes), all of which were fully recovered. There was no serious AE attributed to insulin glargine. Body weight change was significantly different between SU users and nonusers (1.5±2.5 kg vs. −0.9±6.0 kg, P=0.011).
CONCLUSION
The combination add-on therapy of insulin glargine, on metformin and DPP-4 inhibitors with or without SU was safe and efficient in reducing HbA1c levels and thus, is a preferable option in managing T2DM patients exhibiting dysglycemia despite the use of OADs.
Keyword
MeSH Terms
Figure
Reference
-
1. Stumvoll M, Goldstein BJ, van Haeften TW. Pathogenesis of type 2 diabetes. Endocr Res. 2007; 32:19–37. PMID: 18271503.
Article2. Inzucchi SE, Bergenstal RM, Buse JB, Diamant M, Ferrannini E, Nauck M, Peters AL, Tsapas A, Wender R, Matthews DR. Management of hyperglycemia in type 2 diabetes, 2015: a patient-centered approach: update to a position statement of the American Diabetes Association and the European Association for the Study of Diabetes. Diabetes Care. 2015; 38:140–149. PMID: 25538310.
Article3. Esposito K, Cozzolino D, Bellastella G, Maiorino MI, Chiodini P, Ceriello A, Giugliano D. Dipeptidyl peptidase-4 inhibitors and HbA1c target of <7% in type 2 diabetes: meta-analysis of randomized controlled trials. Diabetes Obes Metab. 2011; 13:594–603. PMID: 21320267.4. Ahren B, Landin-Olsson M, Jansson PA, Svensson M, Holmes D, Schweizer A. Inhibition of dipeptidyl peptidase-4 reduces glycemia, sustains insulin levels, and reduces glucagon levels in type 2 diabetes. J Clin Endocrinol Metab. 2004; 89:2078–2084. PMID: 15126524.5. Gallwitz B. Extra-pancreatic effects of incretin-based therapies. Endocrine. 2014; 47:360–371. PMID: 24604239.
Article6. Makimattila S, Nikkila K, Yki-Jarvinen H. Causes of weight gain during insulin therapy with and without metformin in patients with type II diabetes mellitus. Diabetologia. 1999; 42:406–412. PMID: 10230643.
Article7. Charbonnel B, Schweizer A, Dejager S. Combination therapy with DPP-4 inhibitors and insulin in patients with type 2 diabetes mellitus: what is the evidence? Hosp Pract (1995). 2013; 41:93–107. PMID: 23680741.
Article8. Vora J. Combining incretin-based therapies with insulin: realizing the potential in type 2 diabetes. Diabetes Care. 2013; 36(Suppl 2):S226–S232. PMID: 23882050.9. Barnett AH, Charbonnel B, Li J, Donovan M, Fleming D, Iqbal N. Saxagliptin add-on therapy to insulin with or without metformin for type 2 diabetes mellitus: 52-week safety and efficacy. Clin Drug Investig. 2013; 33:707–717.
Article10. Fonseca V, Schweizer A, Albrecht D, Baron MA, Chang I, Dejager S. Addition of vildagliptin to insulin improves glycaemic control in type 2 diabetes. Diabetologia. 2007; 50:1148–1155. PMID: 17387446.
Article11. Rosenstock J, Rendell MS, Gross JL, Fleck PR, Wilson CA, Mekki Q. Alogliptin added to insulin therapy in patients with type 2 diabetes reduces HbA(1C) without causing weight gain or increased hypoglycaemia. Diabetes Obes Metab. 2009; 11:1145–1152. PMID: 19758359.12. Vilsboll T, Rosenstock J, Yki-Jarvinen H, Cefalu WT, Chen Y, Luo E, Musser B, Andryuk PJ, Ling Y, Kaufman KD, Amatruda JM, Engel SS, Katz L. Efficacy and safety of sitagliptin when added to insulin therapy in patients with type 2 diabetes. Diabetes Obes Metab. 2010; 12:167–177. PMID: 20092585.13. Barnett AH. Insulin glargine in the treatment of type 1 and type 2 diabetes. Vasc Health Risk Manag. 2006; 2:59–67. PMID: 17319470.
Article14. Meneghini LF. Early insulin treatment in type 2 diabetes: what are the pros? Diabetes Care. 2009; 32(Suppl 2):S266–S269. PMID: 19875562.15. Holman RR, Paul SK, Bethel MA, Matthews DR, Neil HA. 10-year follow-up of intensive glucose control in type 2 diabetes. N Engl J Med. 2008; 359:1577–1589. PMID: 18784090.
Article16. Unger RH, Grundy S. Hyperglycaemia as an inducer as well as a consequence of impaired islet cell function and insulin resistance: implications for the management of diabetes. Diabetologia. 1985; 28:119–121. PMID: 3888754.
Article17. Meneghini L, Kesavadev J, Demissie M, Nazeri A, Hollander P. Once-daily initiation of basal insulin as add-on to metformin: a 26-week, randomized, treat-to-target trial comparing insulin detemir with insulin glargine in patients with type 2 diabetes. Diabetes Obes Metab. 2013; 15:729–736. PMID: 23421331.
Article18. Hollander P, Raslova K, Skjoth TV, Rastam J, Liutkus JF. Efficacy and safety of insulin detemir once daily in combination with sitagliptin and metformin: the TRANSITION randomized controlled trial. Diabetes Obes Metab. 2011; 13:268–275. PMID: 21205123.
Article19. Rosenstock J, Schwartz SL, Clark CM Jr, Park GD, Donley DW, Edwards MB. Basal insulin therapy in type 2 diabetes: 28-week comparison of insulin glargine (HOE 901) and NPH insulin. Diabetes Care. 2001; 24:631–636. PMID: 11315821.20. Nathan DM, Buse JB, Davidson MB, Ferrannini E, Holman RR, Sherwin R, Zinman B. American Diabetes Association. European Association for Study of Diabetes. Medical management of hyperglycemia in type 2 diabetes: a consensus algorithm for the initiation and adjustment of therapy: a consensus statement of the American Diabetes Association and the European Association for the Study of Diabetes. Diabetes Care. 2009; 32:193–203. PMID: 18945920.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- DPP-4 Inhibitors as a New Option for the Management of Type 2 Diabetes
- Sodium Glucose Cotransporter-2 Inhibitors as an Add-on Therapy to Metformin Plus Dipeptidyl Peptidase-4 Inhibitor in Patients with Type 2 Diabetes
- DPP-4 Inhibitors and the Relations between Rosiglitazone and the Risk of Myocardial Infarction
- Predetermined Anti-Diabetic Drug Regimen Adjustments during Ramadan Fasting: An Observational Study of Safety
- Exenatide versus Insulin Lispro Added to Basal Insulin in a Subgroup of Korean Patients with Type 2 Diabetes Mellitus