Clin Exp Vaccine Res.
2019 Jul;8(2):94-102. 10.7774/cevr.2019.8.2.94.
One-year antibody persistence and safety of a 4-dose schedule of MenACWY-CRM in healthy infants from South Korea
- Affiliations
-
- 1Department of Pediatrics, Seoul National University College of Medicine, Seoul National University Children's Hospital, Seoul, Korea.
- 2Department of Pediatrics, Chonbuk National University Medical School, Chonbuk National University Children's Hospital, Jeonju, Korea.
- 3Department of Pediatrics, Korea University College of Medicine, Korea University Ansan Hospital, Ansan, Korea.
- 4Department of Pediatrics, Seoul National University College of Medicine, Seoul National University Bundang Hospital, Seongnam, Korea.
- 5Department of Pediatrics, Ewha Womans University College of Medicine, Ewha Womans University Mokdong Hospital, Seoul, Korea.
- 6GSK, Seoul, Korea.
- 7GSK, Siena, Italy.
- 8GSK, Amsterdam, The Netherlands. yan.x.miao@gsk.com
- KMID: 2455080
- DOI: http://doi.org/10.7774/cevr.2019.8.2.94
Abstract
- PURPOSE
Results from a post-marketing study to generate evidence on 1-year antibody persistence and safety following vaccination of infants from South Korea with the quadrivalent meningococcal conjugate vaccine MenACWY-CRM.
MATERIALS AND METHODS
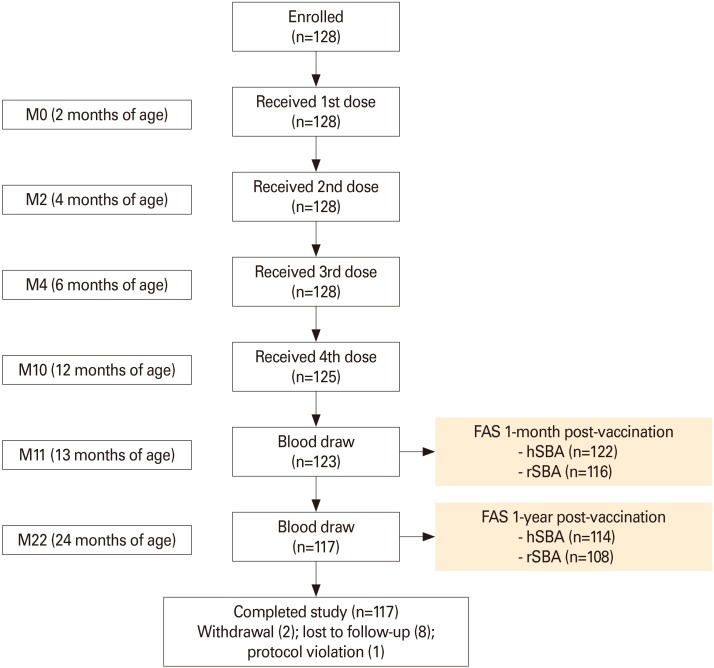
In this phase IV, open-label, multi-center study (NCT02446691), 128 infants received MenACWY-CRM at ages 2, 4, 6, and 12 months. One-year antibody persistence following the full vaccination course was evaluated (primary objective) for the four meningococcal serogroups (Men) by serum bactericidal activity assay using human or rabbit complement (hSBA/rSBA). Immune responses at 1-month post-vaccination and safety were also assessed.
RESULTS
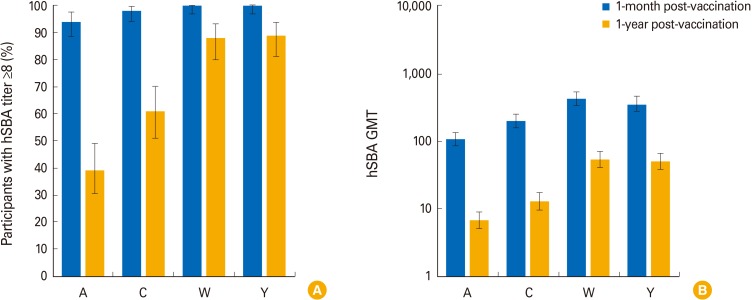
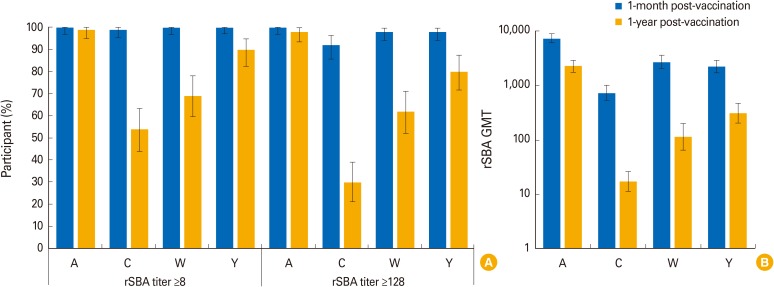
The percentage of children with hSBA titers ≥8 ranged between 94% (MenA) and 100% (MenY/W) 1-month post-vaccination, and from 39% (MenA) to 89% (MenY) 1-year post-vaccination. At least 99% and 92% of children had rSBA titers ≥8 and ≥128 against each meningococcal serogroup, 1-month post-vaccination. One-year post-vaccination, the percentage of children with rSBA titers ≥8 and ≥128 ranged from 54% (MenC) to 99% (MenA) and from 30% (MenC) to 98% (MenA). Geometric mean titers declined from 1-month to 1-year post-vaccination, when they varied between 6.8 (MenA) and 53.6 (MenW) by hSBA and between 17.2 (MenC) and 2,269.5 (MenA) by rSBA. At least one solicited and unsolicited adverse event was reported for 79% and 66% of children. Of 36 serious adverse events reported, none were vaccination-related.
CONCLUSION
Antibody persistence (hSBA/rSBA titers ≥8) was determined in 39%-99% of children 1 year after a 4-dose MenACWY-CRM series during infancy, with an acceptable clinical safety profile.
Keyword
MeSH Terms
Figure
Reference
-
1. Dwilow R, Fanella S. Invasive meningococcal disease in the 21st century: an update for the clinician. Curr Neurol Neurosci Rep. 2015; 15:2. PMID: 25637287.
Article2. Olbrich KJ, Muller D, Schumacher S, Beck E, Meszaros K, Koerber F. Systematic review of invasive meningococcal disease: sequelae and wuality of life impact on patients and their caregivers. Infect Dis Ther. 2018; 7:421–438. PMID: 30267220.3. World Health Organization. Meningococcal meningitis: key facts [Internet]. Geneva: World Health Organization;2018. cited 2019 Apr 24. Available from: http://www.who.int/en/news-room/fact-sheets/detail/meningococcal-meningitis.4. Meningococcal vaccines: WHO position paper, November 2011. Wkly Epidemiol Rec. 2011; 86:521–539. PMID: 22128384.5. Pelton SI. The global evolution of meningococcal epidemiology following the introduction of meningococcal vaccines. J Adolesc Health. 2016; 59(2 Suppl):S3–S11. PMID: 27449148.
Article6. World Health Organization. Invasive meningococcal disease: serogroup distribution [Internet]. Geneva: World Health Organization;2018. cited 2019 Apr 24. Available from: http://www.who.int/emergencies/diseases/meningitis/serogroup-distribution-2018.pdf?ua=1.7. Korea Centers for Disease Control and Prevention. Disease surveillance statistics. Public Health Weekly Report. Vol 12, No. 11 [Internet]. Cheonju: Korea Centers for Disease Control and Prevention;2019. cited 2019 Apr 24. Available from: http://www.cdc.go.kr/CDC/eng/info/Cdc-KeDIDO.jsp?menuIds=HOME002-MNU0576-MNU0583&fid=9712&q_type=&q_value=&cid=143274&pageNum=.8. Kim SA, Kim DW, Dong BQ, Kim JS, Anh DD, Kilgore PE. An expanded age range for meningococcal meningitis: molecular diagnostic evidence from population-based surveillance in Asia. BMC Infect Dis. 2012; 12:310. PMID: 23164061.
Article9. Lee H, Seo Y, Kim KH, Lee K, Choe KW. Prevalence and serogroup changes of Neisseria meningitidis in South Korea, 2010–2016. Sci Rep. 2018; 8:5292. PMID: 29593277.
Article10. Keshavan P, Pellegrini M, Vadivelu-Pechai K, Nissen M. An update of clinical experience with the quadrivalent meningococcal ACWY-CRM conjugate vaccine. Expert Rev Vaccines. 2018; 17:865–880. PMID: 30198805.
Article11. Khatami A, Snape MD, Davis E, et al. Persistence of the immune response at 5 years of age following infant immunisation with investigational quadrivalent MenACWY conjugate vaccine formulations. Vaccine. 2012; 30:2831–2838. PMID: 22394992.
Article12. Klein NP, Block SL, Johnston W, et al. 1085: Persistence of meningococcal bactericidal antibodies and booster response at 60-months of age in children who received infant or toddler doses of MenACWY-CRM conjugate vaccine. Open Forum Infect Dis. 2014; 1(Suppl 1):S319.13. Block SL, Christensen S, Verma B, et al. Antibody persistence 5 years after vaccination at 2 to 10 years of age with Quadrivalent MenACWY-CRM conjugate vaccine, and responses to a booster vaccination. Vaccine. 2015; 33:2175–2182. PMID: 25744224.
Article14. Johnston W, Essink B, Kirstein J, et al. Comparative assessment of a single dose and a 2-dose vaccination series of a quadrivalent meningococcal CRM-conjugate vaccine (MenACWY-CRM) in children 2-10 years of age. Pediatr Infect Dis J. 2016; 35:e19–e27. PMID: 26398741.
Article15. Baxter R, Reisinger K, Block SL, Izu A, Odrljin T, Dull P. Antibody persistence and booster response of a quadrivalent meningococcal conjugate vaccine in adolescents. J Pediatr. 2014; 164:1409–1415. PMID: 24657122.
Article16. Baxter R, Reisinger K, Block SL, et al. Antibody persistence after primary and booster doses of a quadrivalent meningococcal conjugate vaccine in adolescents. Pediatr Infect Dis J. 2014; 33:1169–1176. PMID: 24911896.
Article17. Jacobson RM, Jackson LA, Reisinger K, Izu A, Odrljin T, Dull PM. Antibody persistence and response to a booster dose of a quadrivalent conjugate vaccine for meningococcal disease in adolescents. Pediatr Infect Dis J. 2013; 32:e170–e177. PMID: 23114372.
Article18. Baxter R, Keshavan P, Welsch JA, Han L, Smolenov I. Persistence of the immune response after MenACWY-CRM vaccination and response to a booster dose, in adolescents, children and infants. Hum Vaccin Immunother. 2016; 12:1300–1310. PMID: 26829877.
Article19. Highlights of prescribing information: Menveo [Internet]. Silverspring: U.S. Food and Drug Administration;2018. cited 2019 Apr 24. Available from: http://www.fda.gov/downloads/biologicsbloodvaccines/vaccines/approvedproducts/ucm201349.pdf.20. Maslanka SE, Gheesling LL, Libutti DE, et al. Standardization and a multilaboratory comparison of Neisseria meningitidis serogroup A and C serum bactericidal assays. The Multilaboratory Study Group. Clin Diagn Lab Immunol. 1997; 4:156–167. PMID: 9067649.
Article21. Snape MD, Perrett KP, Ford KJ, et al. Immunogenicity of a tetravalent meningococcal glycoconjugate vaccine in infants: a randomized controlled trial. JAMA. 2008; 299:173–184. PMID: 18182599.
Article22. Clopper CJ, Pearson ES. The use of confidence or fiducial limits illustrated in the case of the binomial. Biometrika. 1934; 26:404–413.
Article23. Findlow J, Balmer P, Borrow R. A review of complement sources used in serum bactericidal assays for evaluating immune responses to meningococcal ACWY conjugate vaccines. Hum Vaccin Immunother. 2019; DOI: 10.1080/21645515.2019.1593082. [Epub].
Article24. Klein NP, Reisinger KS, Johnston W, et al. Safety and immunogenicity of a novel quadrivalent meningococcal CRM-conjugate vaccine given concomitantly with routine vaccinations in infants. Pediatr Infect Dis J. 2012; 31:64–71. PMID: 22094635.
Article25. Block SL, Shepard J, Garfield H, et al. Immunogenicity and safety of a 3- and 4-dose vaccination series of a meningococcal ACWY conjugate vaccine in infants: results of a phase 3b, randomized, open-label trial. Pediatr Infect Dis J. 2016; 35:e48–e59. PMID: 26479973.26. Tregnaghi M, Lopez P, Stamboulian D, et al. Immunogenicity and safety of a quadrivalent meningococcal polysaccharide CRM conjugate vaccine in infants and toddlers. Int J Infect Dis. 2014; 26:22–30. PMID: 24980467.
Article27. Klein NP, Shepard J, Bedell L, Odrljin T, Dull P. Immunogenicity and safety of a quadrivalent meningococcal conjugate vaccine administered concomitantly with measles, mumps, rubella, varicella vaccine in healthy toddlers. Vaccine. 2012; 30:3929–3936. PMID: 22504039.
Article28. Huang LM, Chiu NC, Yeh SJ, Bhusal C, Arora AK. Immunogenicity and safety of a single dose of a CRM-conjugated meningococcal ACWY vaccine in children and adolescents aged 2–18 years in Taiwan: results of an open label study. Vaccine. 2014; 32:5177–5184. PMID: 25075804.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Immunogenicity of MenACWY-CRM in Korean Military Recruits: Influence of Tetanus-Diphtheria Toxoid Vaccination on the Vaccine Response to MenACWY-CRM
- Comparison of Measles Specific IgG in the Sera of Infants and Children after Vaccination of Measles
- Prevalence and Persistence of Transferred Maternal Hepatitis. A Antibody During The Second Year of Life in Korean Infants
- Study on Introduction of Medical CRM into Healthcare
- Immunogenicity and Safety of a Haemophilus influenzae Type b Polysaccharide-Tetanus Toxoid Conjugate Vaccine (PRP-T; Hiberixâ„¢) in Korean Infants