Korean J Ophthalmol.
2019 Aug;33(4):343-352. 10.3341/kjo.2018.0116.
Efficacy of Topical Cyclosporine Nanoemulsion 0.05% Compared with Topical Cyclosporine Emulsion 0.05% and Diquafosol 3% in Dry Eye
- Affiliations
-
- 1Department of Ophthalmology, Yeouido St. Mary's Hospital, The Catholic University of Korea College of Medicine, Seoul, Korea. sara514@catholic.ac.kr
- 2Department of Ophthalmology, Seoul National University Hospital, Seoul National University College of Medicine, Seoul, Korea.
- 3Department of Ophthalmology, Bucheon St. Mary's Hospital, The Catholic University of Korea College of Medicine, Bucheon, Korea.
- 4Department of Ophthalmology, Asan Medical Center, University of Ulsan College of Medicine, Seoul, Korea.
- 5Department of Ophthalmology, Institute of Vision Research, Severance Hospital, Yonsei University College of Medicine, Seoul, Korea.
- 6Department of Ophthalmology, Kyungpook National University School of Medicine, Daegu, Korea.
- 7Department of Ophthalmology, Korea University College of Medicine, Seoul, Korea.
- 8Department of Ophthalmology, Chonnam National University Medical School, Gwangju, Korea.
- 9Department of Ophthalmology, Ilsan Paik Hospital, Inje University College of Medicine, Goyang, Korea.
- 10Department of Ophthalmology, Institute of Vision Research, Gangnam Severance Hospital, Yonsei University College of Medicine, Seoul, Korea.
- 11Department of Ophthalmology, Samsung Medical Center, Sungkyunkwan University School of Medicine, Seoul, Korea.
- 12Department of Ophthalmology, Kangbuk Samsung Hospital, Sungkyunkwan University School of Medicine, Seoul, Korea.
- KMID: 2454775
- DOI: http://doi.org/10.3341/kjo.2018.0116
Abstract
- PURPOSE
To evaluate the efficacy and safety of cyclosporine nanoemulsion 0.05% compared to cyclosporine emulsion 0.05% and diquafosol sodium 3%.
METHODS
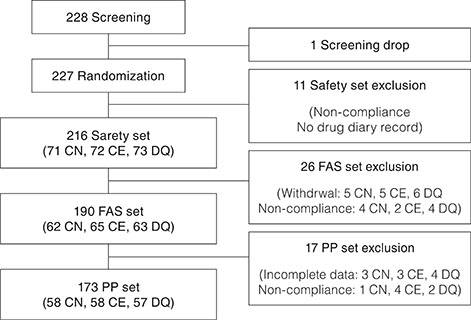
This was a multicenter, randomized, evaluator-masked, active control, parallel, phase IV study. A total of 227 patients were randomly allocated to instill cyclosporine nanoemulsion 0.05% (CN) twice daily, cyclosporine emulsion 0.05% (CE) twice daily, or diquafosol sodium 3% (DQ) six times daily. Non-inferiority of CN was analyzed by primary endpoint (cornea and conjunctival staining scores at week 12). The secondary endpoints were scores of corneal staining, conjunctival staining, tear break-up time, Schirmer test, and Ocular Surface Disease Index at weeks 4 and 12.
RESULTS
Primary endpoints showed statistically significant improvements in all groups. Primary endpoints were −6.60 for the CN group, −5.28 for the CE group, and −6.63 for the DQ group (National Eye Institute scale from 0 to 33), verifying the non-inferiority of CN compared to CE (95% confidence interval, −0.15 to 2.80, Δ>−2.88). In intergroup comparison between CN and CE groups, the CN group had significantly more decreased conjunctival staining score at week 12. Intergroup comparison between CN and DQ groups showed consistent statistically significant improvements in TBUT and Schirmer test in the CN group. In the DQ group, TBUT showed late statistically significant improvement at week 12 and Schirmer test showed relatively short-term statistically significant improvement at week 4.
CONCLUSIONS
Cyclosporine nanoemulsion 0.05% was equivalently efficient compared to cyclosporine emulsion 0.05% and diquafosol sodium 3%. In addition, CN showed significant improvements in several parameters for treatment of dry eyes.
Keyword
Figure
Reference
-
1. Management and therapy of dry eye disease: report of the Management and Therapy Subcommittee of the International Dry Eye WorkShop (2007). Ocul Surf. 2007; 5:163–178.2. Fruman DA, Klee CB, Bierer BE, Burakoff SJ. Calcineurin phosphatase activity in T lymphocytes is inhibited by FK 506 and cyclosporin A. Proc Natl Acad Sci U S A. 1992; 89:3686–3690.
Article3. Yeh S, Song XJ, Farley W, et al. Apoptosis of ocular surface cells in experimentally induced dry eye. Invest Ophthalmol Vis Sci. 2003; 44:124–129.
Article4. Waldmeier PC, Zimmermann K, Qian T, et al. Cyclophilin D as a drug target. Curr Med Chem. 2003; 10:1485–1506.
Article5. Czogalla A. Oral cyclosporine A: the current picture of its liposomal and other delivery systems. Cell Mol Biol Lett. 2009; 14:139–152.
Article6. Lallemand F, Felt-Baeyens O, Besseghir K, et al. Cyclosporine A delivery to the eye: a pharmaceutical challenge. Eur J Pharm Biopharm. 2003; 56:307–318.
Article7. Ran Y, Zhao L, Xu Q, Yalkowsky SH. Solubilization of cyclosporin A. AAPS PharmSciTech. 2001; 2:E2.
Article8. Donnenfeld E, Pflugfelder SC. Topical ophthalmic cyclosporine: pharmacology and clinical uses. Surv Ophthalmol. 2009; 54:321–338.9. Gruner P, Riechers B, Orellana LA, et al. Stabilisers for water-in-fluorinated-oil dispersions: key properties for microfluidic applications. Curr Opin Colloid Interface Sci. 2015; 20:183–191.
Article10. Tang-Liu DD, Acheampong A. Ocular pharmacokinetics and safety of ciclosporin, a novel topical treatment for dry eye. Clin Pharmacokinet. 2005; 44:247–261.
Article11. Thakur A, Walia MK, Kumar SL. Nanoemulsion in enhancement of bioavailability of poorly soluble drugs: a review. Pharmacophore. 2013; 4:15–25.12. Cerpnjak K, Zvonar A, Gasperlin M, Vrecer F. Lipid-based systems as a promising approach for enhancing the bioavailability of poorly water-soluble drugs. Acta Pharm. 2013; 63:427–445.13. Fujihara T, Murakami T, Fujita H, et al. Improvement of corneal barrier function by the P2Y(2) agonist INS365 in a rat dry eye model. Invest Ophthalmol Vis Sci. 2001; 42:96–100.14. Fujihara T, Murakami T, Nagano T, et al. INS365 suppresses loss of corneal epithelial integrity by secretion of mucin-like glycoprotein in a rabbit short-term dry eye model. J Ocul Pharmacol Ther. 2002; 18:363–370.
Article15. Murakami T, Fujihara T, Horibe Y, Nakamura M. Diquafosol elicits increases in net Cl- transport through P2Y2 receptor stimulation in rabbit conjunctiva. Ophthalmic Res. 2004; 36:89–93.16. Keating GM. Diquafosol ophthalmic solution 3 %: a review of its use in dry eye. Drugs. 2015; 75:911–922.17. Takamura E, Tsubota K, Watanabe H, et al. A randomised, double-masked comparison study of diquafosol versus sodium hyaluronate ophthalmic solutions in dry eye patients. Br J Ophthalmol. 2012; 96:1310–1315.
Article18. Lemp MA. Report of the National Eye Institute/Industry workshop on clinical trials in dry eyes. CLAO J. 1995; 21:221–232.19. Craig JP, Nichols KK, Akpek EK, et al. TFOS DEWS II definition and classification report. Ocul Surf. 2017; 15:276–283.
Article20. The definition and classification of dry eye disease: report of the Definition and Classification Subcommittee of the International Dry Eye WorkShop (2007). Ocul Surf. 2007; 5:75–92.21. Stern ME, Beuerman RW, Fox RI, et al. The pathology of dry eye: the interaction between the ocular surface and lacrimal glands. Cornea. 1998; 17:584–589.22. Stevenson W, Chauhan SK, Dana R. Dry eye disease: an immune-mediated ocular surface disorder. Arch Ophthalmol. 2012; 130:90–100.23. Venkatpurwar VP, Rhodes S, Oien KA, et al. Drug- not carrier-dependent haematological and biochemical changes in a repeated dose study of cyclosporine encapsulated polyester nano- and micro-particles: size does not matter. Toxicology. 2015; 330:9–18.
Article24. Yoshida A, Fujihara T, Nakata K. Cyclosporin A increases tear fluid secretion via release of sensory neurotransmitters and muscarinic pathway in mice. Exp Eye Res. 1999; 68:541–546.
Article25. Sall K, Stevenson OD, Mundorf TK, Reis BL. Two multicenter, randomized studies of the efficacy and safety of cyclosporine ophthalmic emulsion in moderate to severe dry eye disease. CsA Phase 3 Study Group Ophthalmology. 2000; 107:631–639.26. Smith RE. The tear film complex: pathogenesis and emerging therapies for dry eyes. Cornea. 2005; 24:1–7.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Comparison of Efficacy and Sensation of Instillation between 0.05% Cyclosporine Nanoemulsion and Microemulsion Type
- Factors Affecting Compliance With 0.05% Cyclosporine Emulsion in Patients With Dry Eye Syndrome
- Study of Utilization Pattern and Compliance with Topical 0.05% Cyclosporine Emulsion in Korean Dry Eye Patients
- The Efficacies and Safeties of a 0.05% Cyclosporine Nanoemulsion and a 0.1% Cyclosporine Cationic Emulsion
- Long-term Evaluation After Topical Cyclosporine Treatment in Dry Eye Patients With Graft-Versus-Host Disease