Cancer Res Treat.
2016 Apr;48(2):848-852. 10.4143/crt.2014.310.
Dermatomyositis in a Patient with Cholangiocarcinoma Detected by an [18F]-Fluorodeoxyglucose Positron Emission Tomography-Computed Tomography Scan
- Affiliations
-
- 1Division of Hematology and Medical Oncology, Department of Internal Medicine, Seoul National University Hospital, Seoul, Korea. kyunghunlee@snu.ac.kr
- 2Division of Rheumatology, Department of Internal Medicine, Seoul National University Hospital, Seoul, Korea.
- 3Department of Pathology, Seoul National University Hospital, Seoul, Korea.
- 4Department of Nuclear Medicine, Seoul National University Hospital, Seoul, Korea.
- 5Cancer Research Institute, Seoul National University, Seoul, Korea.
- KMID: 2454365
- DOI: http://doi.org/10.4143/crt.2014.310
Abstract
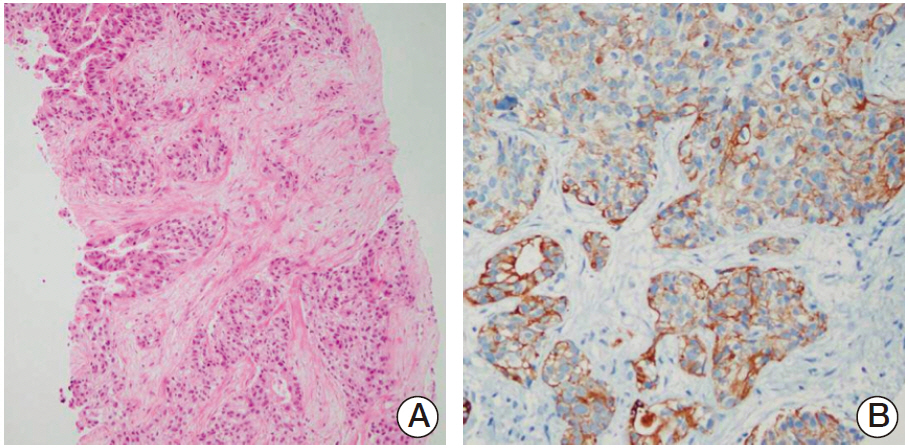
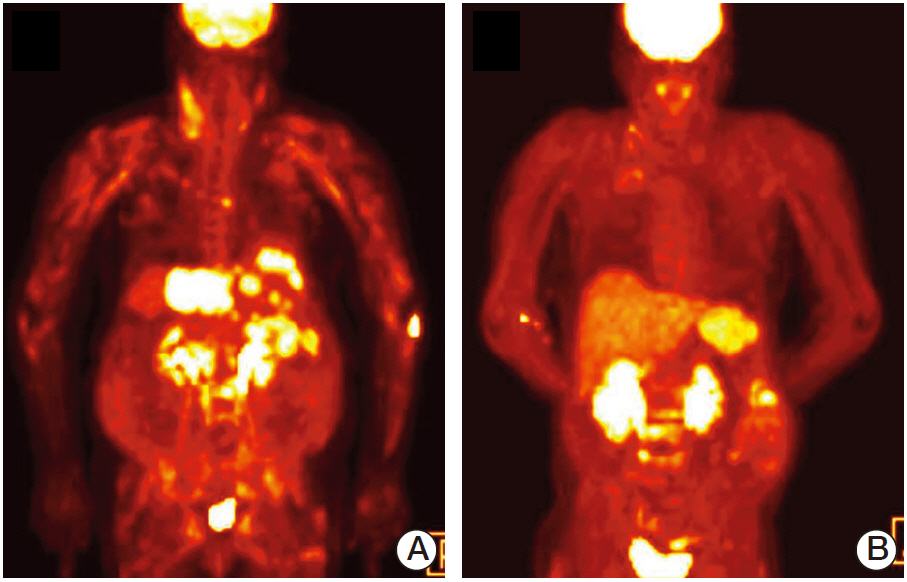
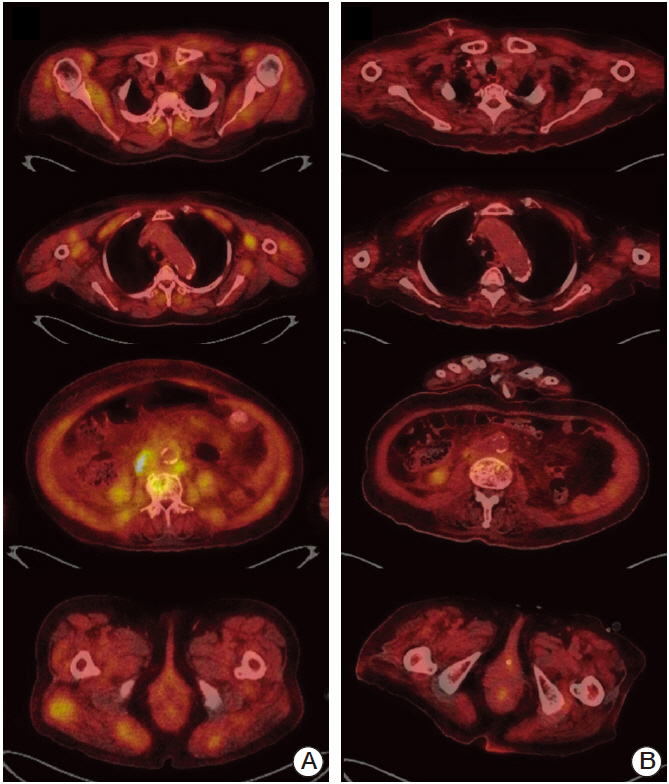
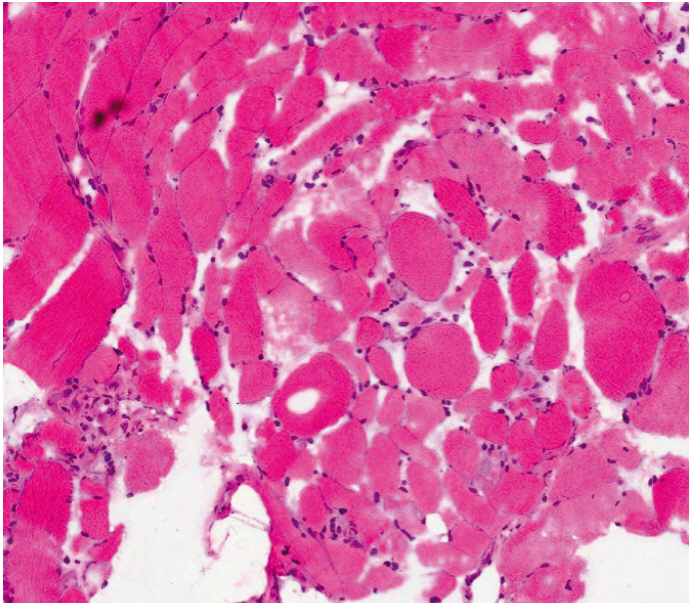
- Cholangiocarcinoma with paraneoplastic dermatomyositis (DM) is extremely rare, and the whole body positron emission tomography-computed tomography (PET-CT) finding of paraneoplastic DM is rarely reported. We report a 66-year-old woman with metastatic cholangiocarcinoma, initially presented with bilateral proximal muscle uptake on PET-CT without clinical muscle symptoms. The initial interpretation of the high muscle uptake was metastasis to the muscles. However, while awaiting for chemotherapy, muscle weakness evolved and rapidly progressed. The level of creatine phosphokinase was significantly elevated. Electromyography revealed moderate myopathy, and a muscle biopsy showed degenerating myofibers with variable sizes. The diagnosis of paraneoplastic dermatomyositis was made. This case highlights that, although rare, paraneoplastic dermatomyositis can be present with cholangiocarcinoma. Also, muscle inflammation can precede the clinical muscle symptoms, and paraneoplastic DM should be considered as a possible differential diagnosis in the assessment of cancer patients who present with abnormal muscle tracer uptake in PET-CT scans.
MeSH Terms
Figure
Reference
-
References
1. Targoff IN. Dermatoniyositis and polymyositis. Curr Probl Dermatol. 1991; 3:134–80.
Article2. Hill CL, Zhang Y, Sigurgeirsson B, Pukkala E, Mellemkjaer L, Airio A, et al. Frequency of specific cancer types in dermatomyositis and polymyositis: a population-based study. Lancet. 2001; 357:96–100.
Article3. Knowles BP, Corcoran NM, Usatoff V. Reading the signs: occult metastatic cholangiocarcinoma detected by full-body screening in dermatomyositis. ANZ J Surg. 2007; 77:1026–7.
Article4. Choi SH, Kim HS, You MR, Kim MW. A case of dermatomyositis associated with infiltrative intrahepatic cholangiocarcinoma. J Korean Rheum Assoc. 2010; 17:76–80.
Article5. Horie Y, Yamada M, Nakai K, Kawasaki H, Hirayama C, Matsui K, et al. Combined hepatocellular-cholangiocarcinoma associated with dermatomyositis. J Gastroenterol Hepatol. 1989; 4:101–4.
Article6. Warburg O. On the origin of cancer cells. Science. 1956; 123:309–14.
Article7. Al-Nahhas A, Jawad AS. PET/CT imaging in inflammatory myopathies. Ann N Y Acad Sci. 2011; 1228:39–45.
Article8. Mummadi V, Arabi M, Jakubowski E, Bou-Assaly W, Geatti O, Gross M, et al. Abnormal 18F-fluorodeoxyglucose (FDG) uptake in muscle tumors on PET-CT: a pictorial atlas of pathologic findings. J Nucl Med. 2010; 51(Suppl 2):1090.9. Casciola-Rosen L, Nagaraju K, Plotz P, Wang K, Levine S, Gabrielson E, et al. Enhanced autoantigen expression in regenerating muscle cells in idiopathic inflammatory myopathy. J Exp Med. 2005; 201:591–601.
Article10. Kaji K, Fujimoto M, Hasegawa M, Kondo M, Saito Y, Komura K, et al. Identification of a novel autoantibody reactive with 155 and 140 kDa nuclear proteins in patients with dermatomyositis: an association with malignancy. Rheumatology (Oxford). 2007; 46:25–8.
Article11. Yoshinaga A, Hayashi T, Ishii N, Ohno R, Watanabe T, Yamada T. Successful cure of dermatomyositis after treatment of nonseminomatous testicular cancer. Int J Urol. 2005; 12:593–5.
Article12. Takahashi F, Tsuta K, Nagaoka T, Miyamoto H, Saito Y, Amano H, et al. Successful resection of dermatomyositis associated with thymic carcinoma: report of a case. Surg Today. 2008; 38:245–8.
Article13. Gunawardena H, Betteridge ZE, McHugh NJ. Myositis-specific autoantibodies: their clinical and pathogenic significance in disease expression. Rheumatology (Oxford). 2009; 48:607–12.
Article14. Noss EH, Hausner-Sypek DL, Weinblatt ME. Rituximab as therapy for refractory polymyositis and dermatomyositis. J Rheumatol. 2006; 33:1021–6.15. Hengstman GJ, ter Laak HJ, Vree Egberts WT, Lundberg IE, Moutsopoulos HM, Vencovsky J, et al. Anti-signal recognition particle autoantibodies: marker of a necrotising myopathy. Ann Rheum Dis. 2006; 65:1635–8.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- 18F-2-Deoxy-2-Fluoro-D-Glucose Positron Emission Tomography: Computed Tomography for Preoperative Staging in Gastric Cancer Patients
- Verification of the Dystonic Muscles Using 18F-Fluorodeoxyglucose Positron Emission Tomography in a Patient with Cervical Dystonia: A case report
- US Findings of Secondary Breast Lymphoma Detected by PET/CT
- A Case of Residual Medullary Thyroid Carcinoma Detected by 18F-FDG-PET/CT in Patient with Persistent Hypercalcitoninemia
- Non-Malignant 18F-FDG Uptake in the Thorax by Positron Emission Tomography Computed Tomography Fusion Imaging