Cancer Res Treat.
2016 Apr;48(2):508-517. 10.4143/crt.2015.172.
18F-FDG/PET May Help to Identify a Subgroup of Patients with T1-T2 Breast Cancer and 1-3 Positive Lymph Nodes Who Are at a High Risk of Recurrence after Mastectomy
- Affiliations
-
- 1Department of Radiation Oncology, Yonsei University College of Medicine, Seoul, Korea. ybkim3@yuhs.ac
- 2Department of Nuclear Medicine, Yonsei University College of Medicine, Seoul, Korea.
- 3Department of Surgery, Yonsei University College of Medicine, Seoul, Korea.
- KMID: 2454327
- DOI: http://doi.org/10.4143/crt.2015.172
Abstract
- PURPOSE
The purpose of this study is to assess the utility of positron emission tomography (PET) for predicting recurrence among patients with T1-T2/N1 breast cancer who were treated with mastectomy.
MATERIALS AND METHODS
Of 712 consecutive patients with T1-T2/N1 breast cancer treated during 2003-2012, 109 had undergone preoperative 18F-fluorodeoxyglucose/PET and were included. Metabolic (maximum standardized uptake value [SUVmax]), volumetric (metabolic tumor volume [MTV]), and combined (total lesion glycolysis [TLG]) indices were measured. The resulting values were analyzed and compared with clinical outcome.
RESULTS
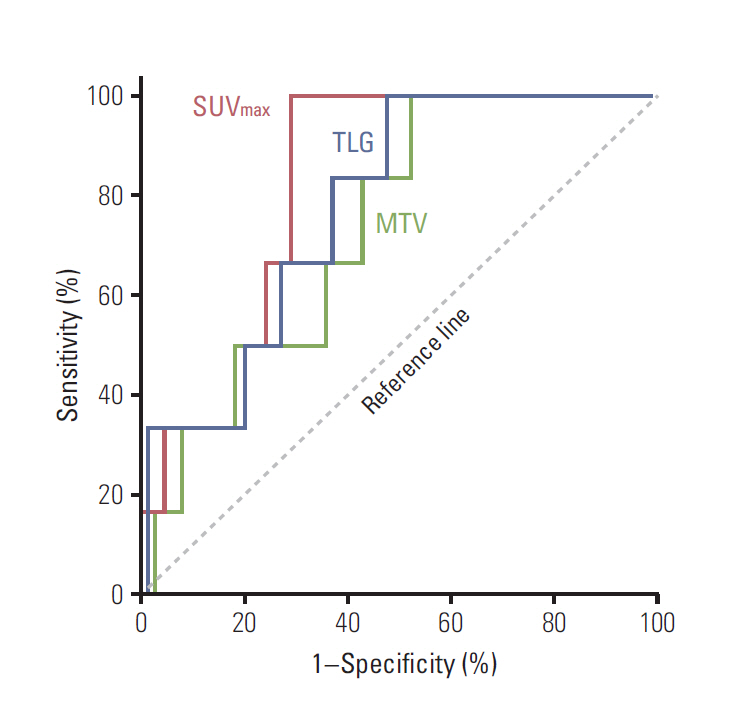
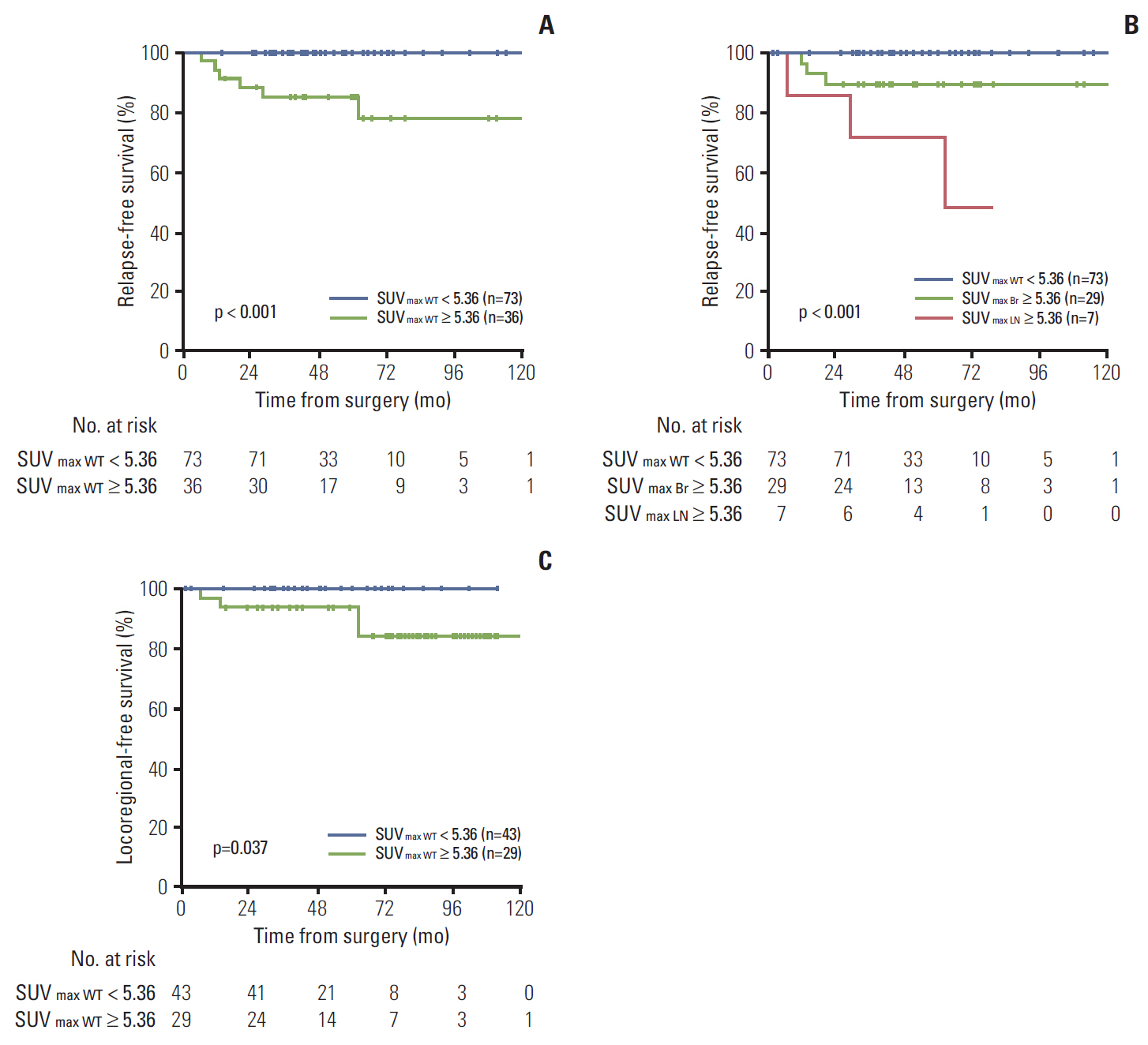
At the median follow-up of 46.7 months, the 3-year relapse-free survival (RFS) rate was 95.2%. SUVmax (area under curve, 0.824) was more useful than MTV or TLG as a means of identifying patients at high risk for any recurrence. In multivariate analysis, SUVmax remained an independent risk factor for RFS (p=0.006). Using the method of Contal and O'Quigley, a SUVmax threshold of 5.36 showed the best predictive performance. The PET-based high-risk group (≥ 5.36 in either breast or nodes) had more T1c-T2, high-grade, hormone-receptor negative, and invasive ductal carcinoma tumors than the low-risk group (< 5.36 in both breast and nodes). The prognosis was much worse when high SUVmax (≥ 5.36) was detected in nodes (p < 0.001). In the no-radiotherapy cohort, the PET-based high-risk group had increased risk of locoregional recurrence when compared to the low-risk group (p=0.037).
CONCLUSION
High SUVmax on preoperative PET showed association with elevated risk of locoregional recurrence and any recurrence. Pre-treatment PET may improve assessments of recurrence risk and clarify indications for post-mastectomy radiotherapy in this subset of patients.
MeSH Terms
Figure
Reference
-
References
1. Clarke M, Collins R, Darby S, Davies C, Elphinstone P, Evans V, et al. Effects of radiotherapy and of differences in the extent of surgery for early breast cancer on local recurrence and 15-year survival: an overview of the randomised trials. Lancet. 2005; 366:2087–106.2. EBCTCG (Early Breast Cancer Trialists' Collaborative Group), McGale P, Taylor C, Correa C, Cutter D, Duane F, et al. Effect of radiotherapy after mastectomy and axillary surgery on 10-year recurrence and 20-year breast cancer mortality: meta-analysis of individual patient data for 8135 women in 22 randomised trials. Lancet. 2014; 383:2127–35.3. Poortmans P. Postmastectomy radiation in breast cancer with one to three involved lymph nodes: ending the debate. Lancet. 2014; 383:2104–6.
Article4. Wallgren A, Bonetti M, Gelber RD, Goldhirsch A, Castiglione-Gertsch M, Holmberg SB, et al. Risk factors for locoregional recurrence among breast cancer patients: results from International Breast Cancer Study Group Trials I through VII. J Clin Oncol. 2003; 21:1205–13.
Article5. Taghian A, Jeong JH, Mamounas E, Anderson S, Bryant J, Deutsch M, et al. Patterns of locoregional failure in patients with operable breast cancer treated by mastectomy and adjuvant chemotherapy with or without tamoxifen and without radiotherapy: results from five National Surgical Adjuvant Breast and Bowel Project randomized clinical trials. J Clin Oncol. 2004; 22:4247–54.
Article6. Langer A. A systematic review of PET and PET/CT in oncology: a way to personalize cancer treatment in a cost-effective manner? BMC Health Serv Res. 2010; 10:283.
Article7. Yun M, Lim JS, Noh SH, Hyung WJ, Cheong JH, Bong JK, et al. Lymph node staging of gastric cancer using (18)F-FDG PET: a comparison study with CT. J Nucl Med. 2005; 46:1582–8.8. Liao S, Penney BC, Wroblewski K, Zhang H, Simon CA, Kampalath R, et al. Prognostic value of metabolic tumor burden on 18F-FDG PET in nonsurgical patients with non-small cell lung cancer. Eur J Nucl Med Mol Imaging. 2012; 39:27–38.
Article9. Contal C, O'Quigley J. An application of changepoint methods in studying the effect of age on survival in breast cancer. Comput Stat Data Anal. 1999; 30:253–70.
Article10. Osborne JR, Port E, Gonen M, Doane A, Yeung H, Gerald W, et al. 18F-FDG PET of locally invasive breast cancer and association of estrogen receptor status with standardized uptake value: microarray and immunohistochemical analysis. J Nucl Med. 2010; 51:543–50.
Article11. Mavi A, Cermik TF, Urhan M, Puskulcu H, Basu S, Yu JQ, et al. The effects of estrogen, progesterone, and C-erbB-2 receptor states on 18F-FDG uptake of primary breast cancer lesions. J Nucl Med. 2007; 48:1266–72.
Article12. Baba S, Isoda T, Maruoka Y, Kitamura Y, Sasaki M, Yoshida T, et al. Diagnostic and prognostic value of pretreatment SUV in 18F-FDG/PET in breast cancer: comparison with apparent diffusion coefficient from diffusion-weighted MR imaging. J Nucl Med. 2014; 55:736–42.
Article13. Song BI, Lee SW, Jeong SY, Chae YS, Lee WK, Ahn BC, et al. 18F-FDG uptake by metastatic axillary lymph nodes on pretreatment PET/CT as a prognostic factor for recurrence in patients with invasive ductal breast cancer. J Nucl Med. 2012; 53:1337–44.
Article14. Nakajima N, Kataoka M, Sugawara Y, Ochi T, Kiyoto S, Ohsumi S, et al. Volume-based parameters of 18F-fluorodeoxyglucose positron emission tomography/computed tomography improve disease recurrence prediction in postmastectomy breast cancer patients with 1 to 3 positive axillary lymph nodes. Int J Radiat Oncol Biol Phys. 2013; 87:738–46.
Article15. Groheux D, Giacchetti S, Espie M, Vercellino L, Hamy AS, Delord M, et al. The yield of 18F-FDG PET/CT in patients with clinical stage IIA, IIB, or IIIA breast cancer: a prospective study. J Nucl Med. 2011; 52:1526–34.
Article16. Pritchard KI, Julian JA, Holloway CM, McCready D, Gulenchyn KY, George R, et al. Prospective study of 2-[(1) (8)F]fluorodeoxyglucose positron emission tomography in the assessment of regional nodal spread of disease in patients with breast cancer: an Ontario clinical oncology group study. J Clin Oncol. 2012; 30:1274–9.17. Groves AM, Shastry M, Ben-Haim S, Kayani I, Malhotra A, Davidson T, et al. Defining the role of PET-CT in staging early breast cancer. Oncologist. 2012; 17:613–9.
Article18. Riedl CC, Slobod E, Jochelson M, Morrow M, Goldman DA, Gonen M, et al. Retrospective analysis of 18F-FDG PET/CT for staging asymptomatic breast cancer patients younger than 40 years. J Nucl Med. 2014; 55:1578–83.
Article19. Groheux D, Moretti JL, Baillet G, Espie M, Giacchetti S, Hindie E, et al. Effect of (18)F-FDG PET/CT imaging in patients with clinical Stage II and III breast cancer. Int J Radiat Oncol Biol Phys. 2008; 71:695–704.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- 18F-FDG PET/CT Findings in a Breast Cancer Patient with Concomitant Tuberculous Axillary Lymphadenitis
- Preoperative Axillary Staging Using 18F-FDG PET/CT and Ultrasonography in Breast Cancer Patients
- (18)F-FDG PET/CT with Contrast Enhancement for Evaluation of Axillary Lymph Node Involvement in T1 Breast Cancer
- Supraclavicular Lymph Node Metastasis from Various Malignancies: Assessment with 18F-Fluorodeoxyglucose Positron Emission Tomography/CT, Contrast-Enhanced CT and Ultrasound
- The Role of fluorodeoxyglucose PET in the management of breast cancer