Clinical Benefit of Maintenance Therapy for Advanced Biliary Tract Cancer Patients Showing No Progression after First-Line Gemcitabine Plus Cisplatin
- Affiliations
-
- 1Department of Oncology, Asan Medical Center, University of Ulsan College of Medicine, Seoul, Korea. kkp1122@amc.seoul.kr, yooc@amc.seoul.kr
- 2Department of Internal Medicine, Hallym University Medical Center, Hallym University College of Medicine, Seoul, Korea.
- KMID: 2454282
- DOI: http://doi.org/10.4143/crt.2018.326
Abstract
- PURPOSE
Gemcitabine plus cisplatin (GemCis) is the standard first-line chemotherapy for patients with advanced biliary tract cancer (BTC). In ABC-02 study, the BTC patients received up to 6-8 cycles of 3-weekly GemCis; however, those without progression often receive more than 6-8 cycles. The clinical benefit of maintenance treatment in patients without progression is uncertain.
MATERIALS AND METHODS
Advanced BTC patients treated with GemCis between April 2010 and February 2015 at Asan Medical Center, Seoul, Korea, were retrospectively analysed. The patients without progression after 6-8 cycles were stratified according to further treatment i.e., with or without further cycles of GemCis (maintenance vs. observation groups). The primary endpoint was overall survival (OS) and progression-free survival (PFS).
RESULTS
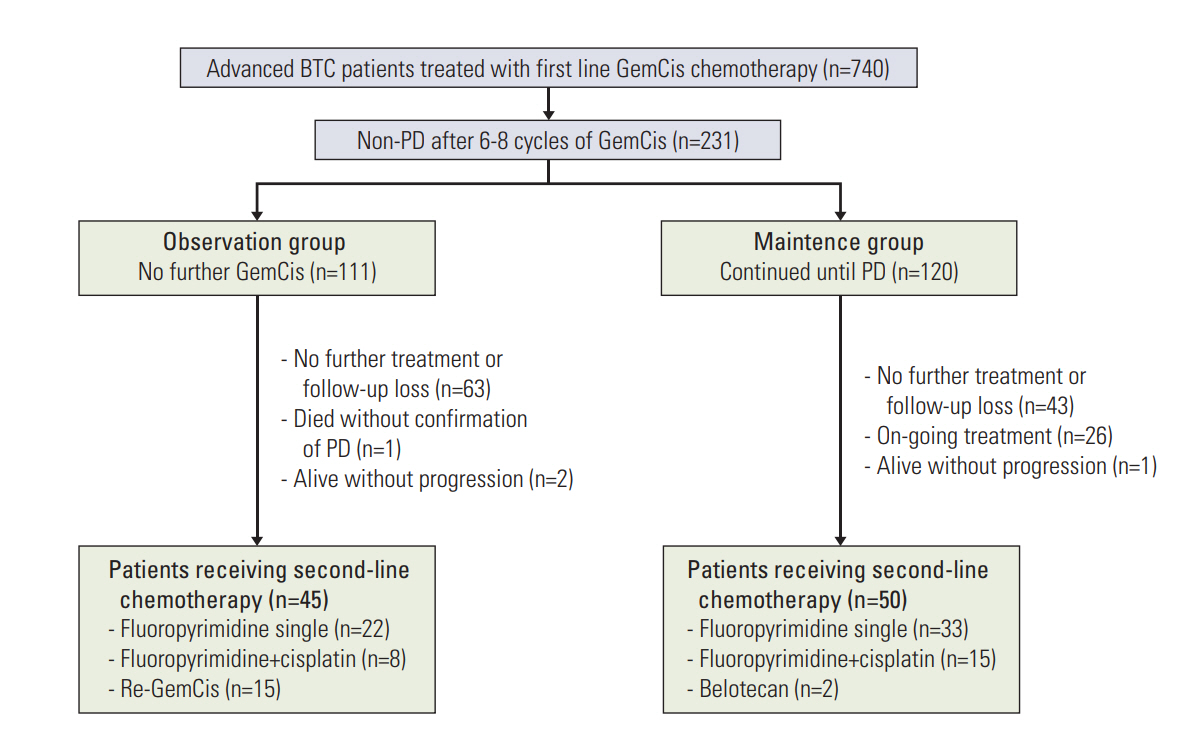
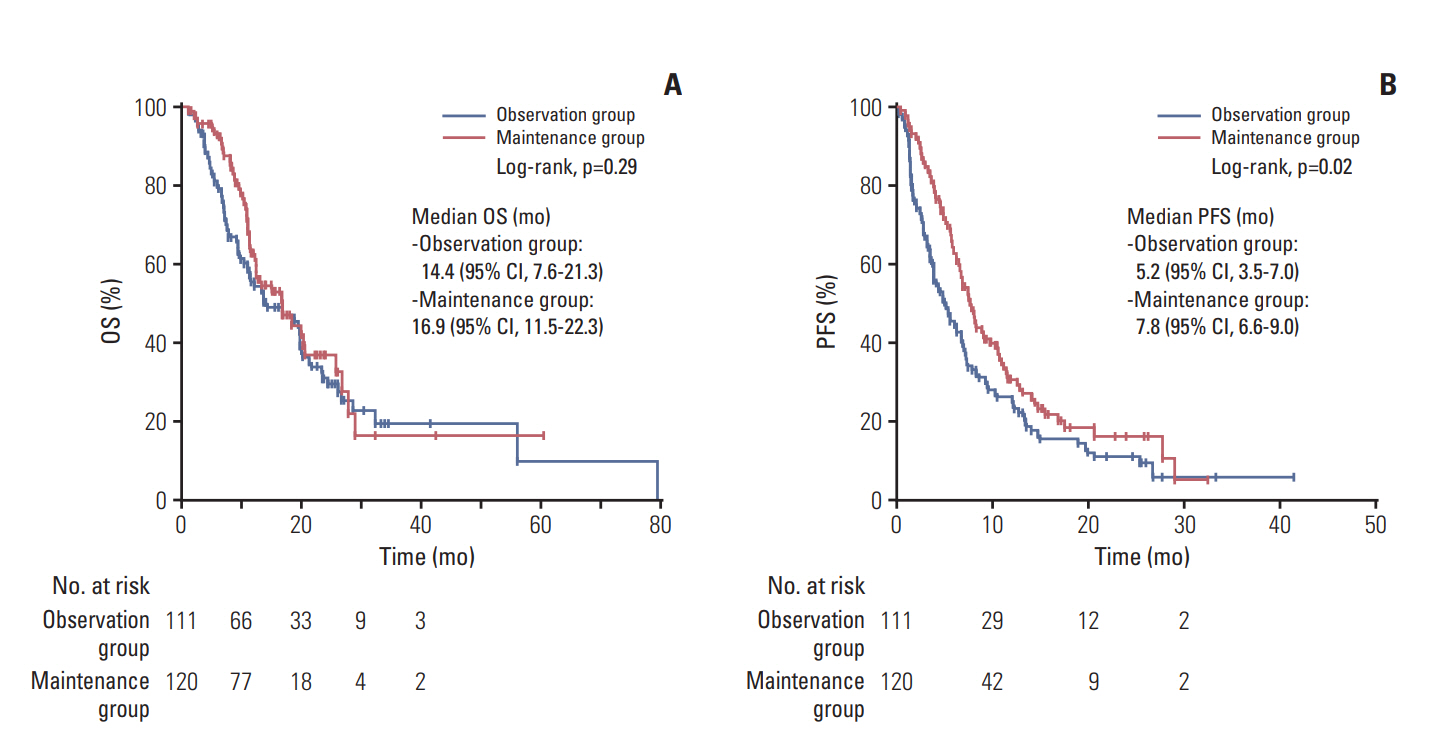
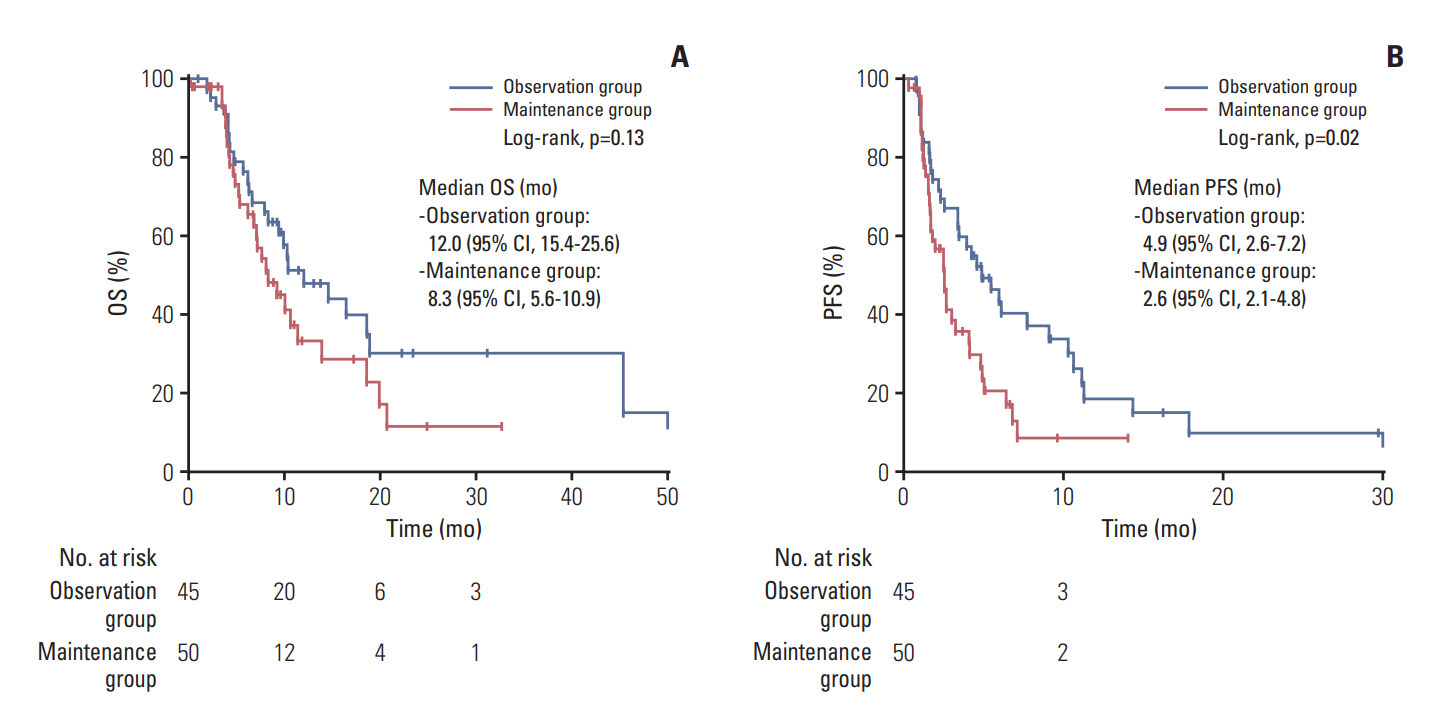
Among the 740 BTC patients in the initial screen, 231 cases (31.2%) were eligible for analysis (111 in the observation group, 120 in the maintenance group). The median OS from the GemCis initiation was 20.5 months (95% confidence interval [CI], 15.4 to 25.6) and 22.4 months (95% CI, 17.0 to 27.8) in the observation and maintenance groups, respectively (p=0.162). The median PFS was 10.4 months (95% CI, 7.0 to 13.8) and 13.2 months (95% CI, 11.3 to 15.2), respectively (p=0.320).
CONCLUSION
sGemCis maintenance is not associated with an improved survival outcome.
MeSH Terms
Figure
Cited by 2 articles
-
Efficacy and Safety of Pembrolizumab in Patients with Refractory Advanced Biliary Tract Cancer: Tumor Proportion Score as a Potential Biomarker for Response
Junho Kang, Jae Ho Jeong, Hee-Sang Hwang, Sang Soo Lee, Do Hyun Park, Dong Wook Oh, Tae Jun Song, Ki-Hun Kim, Shin Hwang, Dae Wook Hwang, Song Cheol Kim, Jin-hong Park, Seung-Mo Hong, Kyu-pyo Kim, Baek-Yeol Ryoo, Changhoon Yoo
Cancer Res Treat. 2020;52(2):594-603. doi: 10.4143/crt.2019.493.Effect of Combining EGFR Tyrosine Kinase Inhibitors and Cytotoxic Agents on Cholangiocarcinoma Cells
Boonyakorn Boonsri, Kiren Yacqub-Usman, Pakpoom Thintharua, Kyaw Zwar Myint, Thannicha Sae-Lao, Pam Collier, Chinnawut Suriyonplengsaeng, Noppadol Larbcharoensub, Brinda Balasubramanian, Simran Venkatraman, Isioma U. Egbuniwe, Dhanwant Gomez, Abhik Mukherjee, Supeecha Kumkate, Tavan Janvilisri, Abed M Zaitoun, Thiti Kuakpaetoon, Rutaiwan Tohtong, Anna M Grabowska, David O. Bates, Kanokpan Wongprasert
Cancer Res Treat. 2021;53(2):457-470. doi: 10.4143/crt.2020.585.
Reference
-
References
1. Patel T. Increasing incidence and mortality of primary intrahepatic cholangiocarcinoma in the United States. Hepatology. 2001; 33:1353–7.
Article2. Siegel RL, Miller KD, Jemal A. Cancer statistics, 2017. CA Cancer J Clin. 2017; 67:7–30.
Article3. Howlader N, Noone AM, Krapcho M, Miller D, Bishop K, Altekruse SF, et al. SEER cancer statistics review, 1975-2013. Bethesda, MD: National Cancer Institute;2016.4. Edge SB, Compton CC. The American Joint Committee on Cancer: the 7th edition of the AJCC cancer staging manual and the future of TNM. Ann Surg Oncol. 2010; 17:1471–4.
Article5. Valle J, Wasan H, Palmer DH, Cunningham D, Anthoney A, Maraveyas A, et al. Cisplatin plus gemcitabine versus gemcitabine for biliary tract cancer. N Engl J Med. 2010; 362:1273–81.
Article6. Kim BJ, Yoo C, Kim KP, Hyung J, Park SJ, Ryoo BY, et al. Efficacy of fluoropyrimidine-based chemotherapy in patients with advanced biliary tract cancer after failure of gemcitabine plus cisplatin: retrospective analysis of 321 patients. Br J Cancer. 2017; 116:561–7.
Article7. Ostwal V, Pinninti R, Ramaswamy A, Shetty N, Goel M, Patkar S, et al. Treatment of advanced Gall bladder cancer in the real world-can continuation chemotherapy improve outcomes? J Gastrointest Oncol. 2017; 8:368–76.
Article8. Maughan TS, James RD, Kerr DJ, Ledermann JA, Seymour MT, Topham C, et al. Comparison of intermittent and continuous palliative chemotherapy for advanced colorectal cancer: a multicentre randomised trial. Lancet. 2003; 361:457–64.
Article9. Tonini G, Imperatori M, Vincenzi B, Frezza AM, Santini D. Rechallenge therapy and treatment holiday: different strategies in management of metastatic colorectal cancer. J Exp Clin Cancer Res. 2013; 32:92.
Article10. Tournigand C, Cervantes A, Figer A, Lledo G, Flesch M, Buyse M, et al. OPTIMOX1: a randomized study of FOLFOX4 or FOLFOX7 with oxaliplatin in a stop-and-Go fashion in advanced colorectal cancer--a GERCOR study. J Clin Oncol. 2006; 24:394–400.
Article11. Labianca R, Sobrero A, Isa L, Cortesi E, Barni S, Nicolella D, et al. Intermittent versus continuous chemotherapy in advanced colorectal cancer: a randomised 'GISCAD' trial. Ann Oncol. 2011; 22:1236–42.
Article12. Park JO, Kim SW, Ahn JS, Suh C, Lee JS, Jang JS, et al. Phase III trial of two versus four additional cycles in patients who are nonprogressive after two cycles of platinum-based chemotherapy in non small-cell lung cancer. J Clin Oncol. 2007; 25:5233–9.13. Paz-Ares LG, de Marinis F, Dediu M, Thomas M, Pujol JL, Bidoli P, et al. PARAMOUNT: Final overall survival results of the phase III study of maintenance pemetrexed versus placebo immediately after induction treatment with pemetrexed plus cisplatin for advanced nonsquamous non-small-cell lung cancer. J Clin Oncol. 2013; 31:2895–902.
Article14. Park YH, Jung KH, Im SA, Sohn JH, Ro J, Ahn JH, et al. Phase III, multicenter, randomized trial of maintenance chemotherapy versus observation in patients with metastatic breast cancer after achieving disease control with six cycles of gemcitabine plus paclitaxel as first-line chemotherapy: KCSG-BR07-02. J Clin Oncol. 2013; 31:1732–9.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Current status of chemotherapy for the treatment of advanced biliary tract cancer
- Novel Palliative Chemotherapy for Cholangiocarcinoma
- Chemotherapy for Biliary Tract Cancer
- Long Term Complete Response of Unresectable Locally Advanced Pancreatic Cancer after CCRT and Gemcitabine Chemotherapy
- A Single-Arm Phase II Study of Nab-Paclitaxel Plus Gemcitabine and Cisplatin for Locally Advanced or Metastatic Biliary Tract Cancer