Diabetes Metab J.
2019 Jun;43(3):287-301. 10.4093/dmj.2018.0054.
Acarbose Add-on Therapy in Patients with Type 2 Diabetes Mellitus with Metformin and Sitagliptin Failure: A Multicenter, Randomized, Double-Blind, Placebo-Controlled Study
- Affiliations
-
- 1Department of Internal Medicine, College of Medicine, The Catholic University of Korea, Seoul, Korea. yoonk@catholic.ac.kr
- 2Division of Endocrinology and Metabolism, Department of Internal Medicine, Seoul St. Mary's Hospital, College of Medicine, The Catholic University of Korea, Seoul, Korea.
- 3Division of Endocrinology and Metabolism, Department of Internal Medicine, St. Vincent's Hospital, College of Medicine, The Catholic University of Korea, Suwon, Korea.
- 4Division of Endocrinology and Metabolism, Department of Internal Medicine, Severance Hospital, Yonsei University College of Medicine, Seoul, Korea.
- 5Division of Endocrinology and Metabolism, Department of Internal Medicine, Kangbuk Samsung Hospital, Sungkyunkwan University School of Medicine, Seoul, Korea.
- 6Division of Endocrinology and Metabolism, Department of Internal Medicine, Eulji General Hospital, Eulji University School of Medicine, Seoul, Korea.
- KMID: 2454058
- DOI: http://doi.org/10.4093/dmj.2018.0054
Abstract
- BACKGROUND
We evaluated the efficacy and safety of acarbose add-on therapy in Korean patients with type 2 diabetes mellitus (T2DM) who are inadequately controlled with metformin and sitagliptin.
METHODS
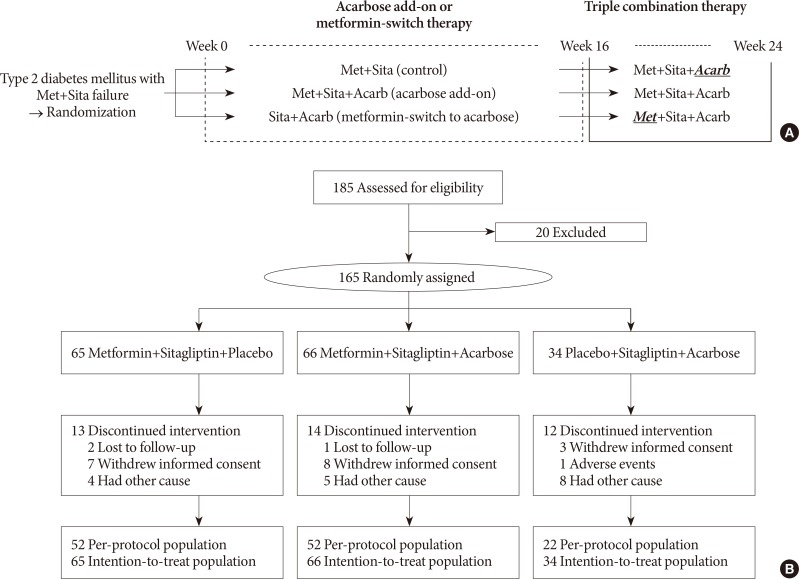
A total of 165 subjects were randomized to metformin and sitagliptin (Met+Sita, n=65), metformin, sitagliptin, and acarbose (Met+Sita+Acarb, n=66) and sitagliptin and acarbose (Sita+Acarb, exploratory assessment, n=34) therapy in five institutions in Korea. After 16 weeks of acarbose add-on or metformin-switch therapy, a triple combination therapy was maintained from week 16 to 24.
RESULTS
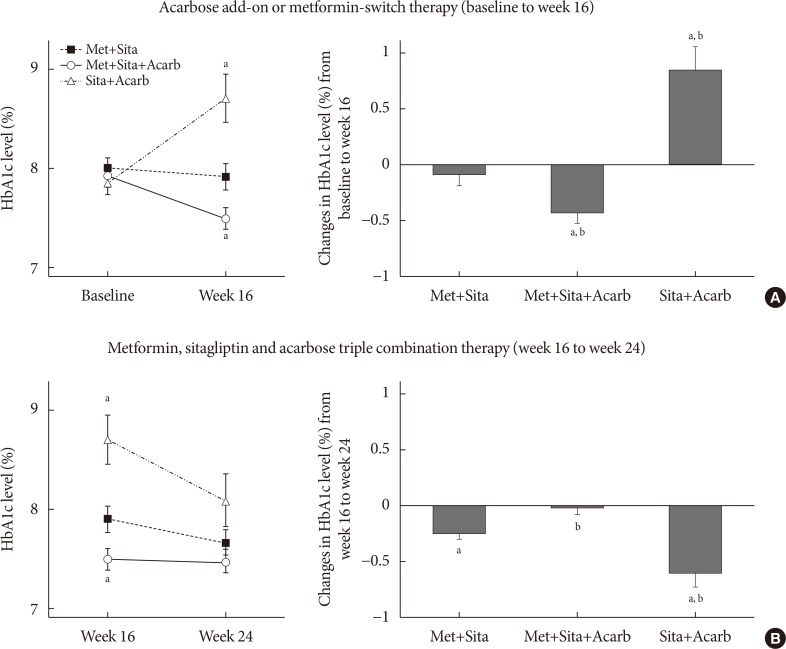
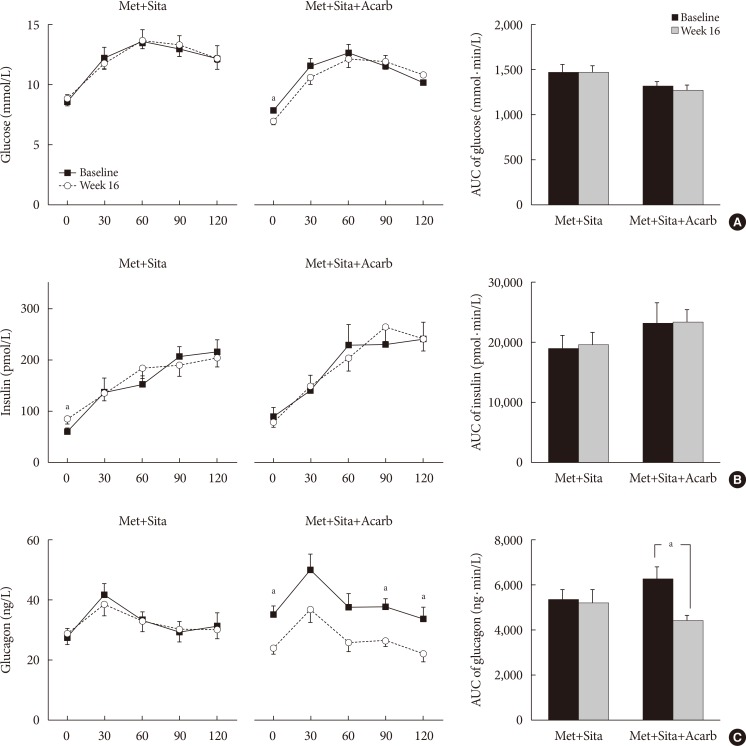
The add-on of acarbose (Met+Sita+Acarb group) demonstrated a 0.44%±0.08% (P<0.001 vs. baseline) decrease in glycosylated hemoglobin (HbA1c) at week 16, while changes in HbA1c were insignificant in the Met+Sita group (−0.09%±0.10%, P=0.113). After 8 weeks of triple combination therapy, HbA1c levels were comparable between Met+Sita and Met+Sita+Acarb group (7.66%±0.13% vs. 7.47%±0.12%, P=0.321). Acarbose add-on therapy demonstrated suppressed glucagon secretion (area under the curve of glucagon, 4,726.17±415.80 ng·min/L vs. 3,314.38±191.63 ng·min/L, P=0.004) in the absence of excess insulin secretion during the meal tolerance tests at week 16 versus baseline. The incidence of adverse or serious adverse events was similar between two groups.
CONCLUSION
In conclusion, a 16-week acarbose add-on therapy to metformin and sitagliptin, effectively lowered HbA1c without significant adverse events. Acarbose might be a good choice as a third-line therapy in addition to metformin and sitagliptin in Korean subjects with T2DM who have predominant postprandial hyperglycemia and a high carbohydrate intake.
Keyword
MeSH Terms
Figure
Reference
-
1. Hampp C, Borders-Hemphill V, Moeny DG, Wysowski DK. Use of antidiabetic drugs in the U.S., 2003-2012. Diabetes Care. 2014; 37:1367–1374. PMID: 24623020.
Article2. Inzucchi SE, Bergenstal RM, Buse JB, Diamant M, Ferrannini E, Nauck M, Peters AL, Tsapas A, Wender R, Matthews DR. Management of hyperglycemia in type 2 diabetes, 2015: a patient-centered approach: update to a position statement of the American Diabetes Association and the European Association for the Study of Diabetes. Diabetes Care. 2015; 38:140–149. PMID: 25538310.
Article3. Garber AJ, Abrahamson MJ, Barzilay JI, Blonde L, Bloomgarden ZT, Bush MA, Dagogo-Jack S, Davidson MB, Einhorn D, Garber JR, Garvey WT, Grunberger G, Handelsman Y, Hirsch IB, Jellinger PS, McGill JB, Mechanick JI, Rosenblit PD, Umpierrez G, Davidson MH. AACE/ACE comprehensive diabetes management algorithm 2015. Endocr Pract. 2015; 21:438–447. PMID: 25877012.
Article4. Yang W, Liu J, Shan Z, Tian H, Zhou Z, Ji Q, Weng J, Jia W, Lu J, Liu J, Xu Y, Yang Z, Chen W. Acarbose compared with metformin as initial therapy in patients with newly diagnosed type 2 diabetes: an open-label, non-inferiority randomized trial. Lancet Diabetes Endocrinol. 2014; 2:46–55. PMID: 24622668.5. Sheu WH, Rosman A, Mithal A, Chung N, Lim YT, Deerochanawong C, Soewondo P, Lee MK, Yoon KH, Schnell O. Addressing the burden of type 2 diabetes and cardiovascular disease through the management of postprandial hyperglycaemia: an Asian-Pacific perspective and expert recommendations. Diabetes Res Clin Pract. 2011; 92:312–321. PMID: 21605924.
Article6. Wang JS, Tu ST, Lee IT, Lin SD, Lin SY, Su SL, Lee WJ, Sheu WH. Contribution of postprandial glucose to excess hyperglycaemia in Asian type 2 diabetic patients using continuous glucose monitoring. Diabetes Metab Res Rev. 2011; 27:79–84. PMID: 21218511.
Article7. Narita T, Katsuura Y, Sato T, Hosoba M, Fujita H, Morii T, Yamada Y. Miglitol induces prolonged and enhanced glucagon-like peptide-1 and reduced gastric inhibitory polypeptide responses after ingestion of a mixed meal in Japanese type 2 diabetic patients. Diabet Med. 2009; 26:187–188. PMID: 19236625.8. Horikawa Y, Enya M, Iizuka K, Chen GY, Kawachi S, Suwa T, Takeda J. Synergistic effect of α-glucosidase inhibitors and dipeptidyl peptidase 4 inhibitor treatment. J Diabetes Investig. 2011; 2:200–203.
Article9. Moritoh Y, Takeuchi K, Hazama M. Combination treatment with alogliptin and voglibose increases active GLP-1 circulation, prevents the development of diabetes and preserves pancreatic beta-cells in prediabetic db/db mice. Diabetes Obes Metab. 2010; 12:224–233. PMID: 20151999.10. Tajima N, Kadowaki T, Okamoto T, Sato A, Okuyama K, Minamide T, Arjona Ferreira JC. Sitagliptin added to voglibose monotherapy improves glycemic control in patients with type 2 diabetes. J Diabetes Investig. 2013; 4:595–604.
Article11. Masuda K, Aoki K, Kamiko K, Takihata M, Ito Y, Nagakura M, Kawasaki S, Akema N, Hasegawa M, Nakajima S, Shinoda K, Toumura S, Tsunoda S, Enomoto H, Shimotomai H, Terauchi Y. Glycemic control after addition of the dipeptidyl peptidase-4 inhibitor alogliptin in patients with type 2 diabetes showing inadequate response to thrice-a-day treatment with α-glucosidase inhibitors. Expert Opin Pharmacother. 2013; 14:1111–1118. PMID: 23600363.
Article12. Seino Y, Fujita T, Hiroi S, Hirayama M, Kaku K. Alogliptin plus voglibose in Japanese patients with type 2 diabetes: a randomized, double-blind, placebo-controlled trial with an open-label, long-term extension. Curr Med Res Opin. 2011; 27(Suppl 3):21–29. PMID: 22106975.
Article13. Min SH, Yoon JH, Hahn S, Cho YM. Efficacy and safety of combination therapy with an α-glucosidase inhibitor and a dipeptidyl peptidase-4 inhibitor in patients with type 2 diabetes mellitus: A systematic review with meta-analysis. J Diabetes Investig. 2018; 9:893–902.
Article14. Kurozumi A, Okada Y, Mori H, Arao T, Tanaka Y. Efficacy of α-glucosidase inhibitors combined with dipeptidyl-peptidase-4 inhibitor (alogliptin) for glucose fluctuation in patients with type 2 diabetes mellitus by continuous glucose monitoring. J Diabetes Investig. 2013; 4:393–398.15. Aoki K, Kamiyama H, Yoshimura K, Shibuya M, Masuda K, Terauchi Y. Miglitol administered before breakfast increased plasma active glucagon-like peptide-1 (GLP-1) levels after lunch in patients with type 2 diabetes treated with sitagliptin. Acta Diabetol. 2012; 49:225–230. PMID: 21898126.
Article16. Kusunoki Y, Katsuno T, Myojin M, Miyakoshi K, Ikawa T, Matsuo T, Ochi F, Tokuda M, Murai K, Miuchi M, Hamaguchi T, Miyagawa J, Namba M. Effect of additional administration of acarbose on blood glucose fluctuations and postprandial hyperglycemia in patients with type 2 diabetes mellitus under treatment with alogliptin. Endocr J. 2013; 60:431–439. PMID: 23220949.
Article17. Service FJ, Molnar GD, Rosevear JW, Ackerman E, Gatewood LC, Taylor WF. Mean amplitude of glycemic excursions, a measure of diabetic instability. Diabetes. 1970; 19:644–655. PMID: 5469118.
Article18. Lam KS, Tiu SC, Tsang MW, Ip TP, Tam SC. Acarbose in NIDDM patients with poor control on conventional oral agents. A 24-week placebo-controlled study. Diabetes Care. 1998; 21:1154–1158. PMID: 9653611.
Article19. Turner RC, Cull CA, Frighi V, Holman RR. UK Prospective Diabetes Study (UKPDS) Group. Glycemic control with diet, sulfonylurea, metformin, or insulin in patients with type 2 diabetes mellitus: progressive requirement for multiple therapies (UKPDS 49). JAMA. 1999; 281:2005–2012. PMID: 10359389.20. Lee YK, Song SO, Kim KJ, Cho Y, Choi Y, Yun Y, Lee BW, Kang ES, Cha BS, Lee HC. Glycemic effectiveness of metformin-based dual-combination therapies with sulphonylurea, pioglitazone, or DPP4-inhibitor in drug-naïve Korean type 2 diabetic patients. Diabetes Metab J. 2013; 37:465–474. PMID: 24404518.
Article21. Mearns ES, Saulsberry WJ, White CM, Kohn CG, Lemieux S, Sihabout A, Salamucha I, Coleman CI. Efficacy and safety of antihyperglycaemic drug regimens added to metformin and sulphonylurea therapy in type 2 diabetes: a network meta-analysis. Diabet Med. 2015; 32:1530–1540. PMID: 26104021.
Article22. van de Laar FA, Lucassen PL, Akkermans RP, van de Lisdonk EH, Rutten GE, van Weel C. Alpha-glucosidase inhibitors for patients with type 2 diabetes: results from a Cochrane systematic review and meta-analysis. Diabetes Care. 2005; 28:154–163. PMID: 15616251.23. Monami M, Lamanna C, Marchionni N, Mannucci E. Comparison of different drugs as add-on treatments to metformin in type 2 diabetes: a meta-analysis. Diabetes Res Clin Pract. 2008; 79:196–203. PMID: 17931733.
Article24. Bischoff H. The mechanism of alpha-glucosidase inhibition in the management of diabetes. Clin Invest Med. 1995; 18:303–311. PMID: 8549017.25. Lee A, Patrick P, Wishart J, Horowitz M, Morley JE. The effects of miglitol on glucagon-like peptide-1 secretion and appetite sensations in obese type 2 diabetics. Diabetes Obes Metab. 2002; 4:329–335. PMID: 12190996.
Article26. Aoki K, Masuda K, Miyazaki T, Togashi Y, Terauchi Y. Effects of miglitol, sitagliptin or their combination on plasma glucose, insulin and incretin levels in non-diabetic men. Endocr J. 2010; 57:667–672. PMID: 20519806.
Article27. Mikada A, Narita T, Yokoyama H, Yamashita R, Horikawa Y, Tsukiyama K, Yamada Y. Effects of miglitol, sitagliptin, and initial combination therapy with both on plasma incretin responses to a mixed meal and visceral fat in over-weight Japanese patients with type 2 diabetes. “the MASTER randomized, controlled trial”. Diabetes Res Clin Pract. 2014; 106:538–547. PMID: 25451890.
Article28. Hucking K, Kostic Z, Pox C, Ritzel R, Holst JJ, Schmiegel W, Nauck MA. Alpha-glucosidase inhibition (acarbose) fails to enhance secretion of glucagon-like peptide 1 (7-36 amide) and to delay gastric emptying in type 2 diabetic patients. Diabet Med. 2005; 22:470–476. PMID: 15787675.29. Ueno H, Tsuchimochi W, Wang HW, Yamashita E, Tsubouchi C, Nagamine K, Sakoda H, Nakazato M. Effects of miglitol, acarbose, and sitagliptin on plasma insulin and gut peptides in type 2 diabetes mellitus: a crossover study. Diabetes Ther. 2015; 6:187–196. PMID: 26055217.
Article30. Kishimoto M, Noda M. Additive effects of miglitol and anagliptin on insulin-treated type 2 diabetes mellitus: a case study. Clin Drug Investig. 2015; 35:141–147.
Article31. de Heer J, Rasmussen C, Coy DH, Holst JJ. Glucagon-like peptide-1, but not glucose-dependent insulinotropic peptide, inhibits glucagon secretion via somatostatin (receptor subtype 2) in the perfused rat pancreas. Diabetologia. 2008; 51:2263–2270. PMID: 18795252.
Article32. De Marinis YZ, Salehi A, Ward CE, Zhang Q, Abdulkader F, Bengtsson M, Braha O, Braun M, Ramracheya R, Amisten S, Habib AM, Moritoh Y, Zhang E, Reimann F, Rosengren A, Shibasaki T, Gribble F, Renstrom E, Seino S, Eliasson L, Rorsman P. GLP-1 inhibits and adrenaline stimulates glucagon release by differential modulation of N- and L-type Ca2+ channel-dependent exocytosis. Cell Metab. 2010; 11:543–553. PMID: 20519125.
Article33. Gotoh K, Masaki T, Chiba S, Ando H, Fujiwara K, Shimasaki T, Mitsutomi K, Katsuragi I, Kakuma T, Sakata T, Yoshimatsu H. Hypothalamic brain-derived neurotrophic factor regulates glucagon secretion mediated by pancreatic efferent nerves. J Neuroendocrinol. 2013; 25:302–311. PMID: 23157205.
Article34. Gu Y, Wang X, Li J, Zhang Y, Zhong H, Liu R, Zhang D, Feng Q, Xie X, Hong J, Ren H, Liu W, Ma J, Su Q, Zhang H, Yang J, Wang X, Zhao X, Gu W, Bi Y, Peng Y, Xu X, Xia H, Li F, Xu X, Yang H, Xu G, Madsen L, Kristiansen K, Ning G, Wang W. Analyses of gut microbiota and plasma bile acids enable stratification of patients for antidiabetic treatment. Nat Commun. 2017; 8:1785. PMID: 29176714.
Article35. Bahne E, Hansen M, Bronden A, Sonne DP, Vilsboll T, Knop FK. Involvement of glucagon-like peptide-1 in the glucose-lowering effect of metformin. Diabetes Obes Metab. 2016; 18:955–961. PMID: 27265206.
Article36. Yamaguchi M, Saji T, Mita S, Kulmatycki K, He YL, Furihata K, Sekiguchi K. Pharmacokinetic and pharmacodynamic interaction of vildagliptin and voglibose in Japanese patients with type 2 diabetes. Int J Clin Pharmacol Ther. 2013; 51:641–651. PMID: 23782587.
Article37. Pan C, Yang W, Barona JP, Wang Y, Niggli M, Mohideen P, Wang Y, Foley JE. Comparison of vildagliptin and acarbose monotherapy in patients with type 2 diabetes: a 24-week, double-blind, randomized trial. Diabet Med. 2008; 25:435–441. PMID: 18341596.
Article38. Holman RR, Cull CA, Turner RC. A randomized double-blind trial of acarbose in type 2 diabetes shows improved glycemic control over 3 years (U.K. Prospective Diabetes Study 44). Diabetes Care. 1999; 22:960–964. PMID: 10372249.
Article39. Bloomgarden ZT, Dodis R, Viscoli CM, Holmboe ES, Inzucchi SE. Lower baseline glycemia reduces apparent oral agent glucose-lowering efficacy: a meta-regression analysis. Diabetes Care. 2006; 29:2137–2139. PMID: 16936168.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- The Effect of Acarbose as an Adjuvant Therapy in Sulfonylurea-Treated NIDDM Patients
- The Effect of Vildagliptin on Visfatin in Korean Patients with Type 2 Diabetes: A Multicenter, Double-Blind, Randomized, Placebo-Controlled, Prospective Study
- Letter: Efficacy and Safety of Voglibose Plus Metformin in Patients with Type 2 Diabetes Mellitus: A Randomized Controlled Trial (Diabetes Metab J 2019;43;276-86)
- Efficacy of Sitagliptin When Added to Ongoing Therapy in Korean Subjects with Type 2 Diabetes Mellitus
- Efficacy of Gemigliptin Add-on to Dapagliflozin and Metformin in Type 2 Diabetes Patients: A Randomized, Double-Blind, Placebo-Controlled Study (SOLUTION)