Diabetes Metab J.
2019 Jun;43(3):247-256. 10.4093/dmj.2018.0221.
Mitochondrial Dysfunction in Adipocytes as a Primary Cause of Adipose Tissue Inflammation
- Affiliations
-
- 1Department of Internal Medicine, University of Ulsan College of Medicine, Seoul, Korea. kulee@amc.seoul.kr
- 2Department of Internal Medicine, Inje University Haeundae Paik Hospital, Inje University College of Medicine, Busan, Korea.
- 3Department of Internal Medicine, Dongguk University Ilsan Hospital, Dongguk University College of Medicine, Goyang, Korea.
- KMID: 2454054
- DOI: http://doi.org/10.4093/dmj.2018.0221
Abstract
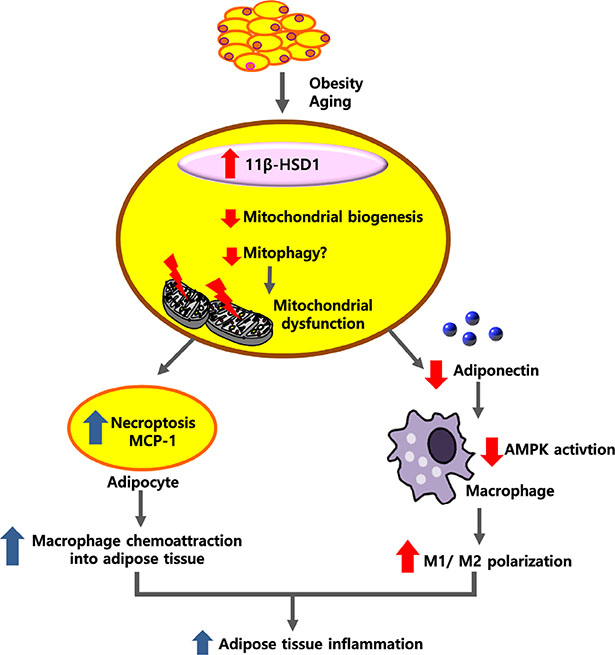
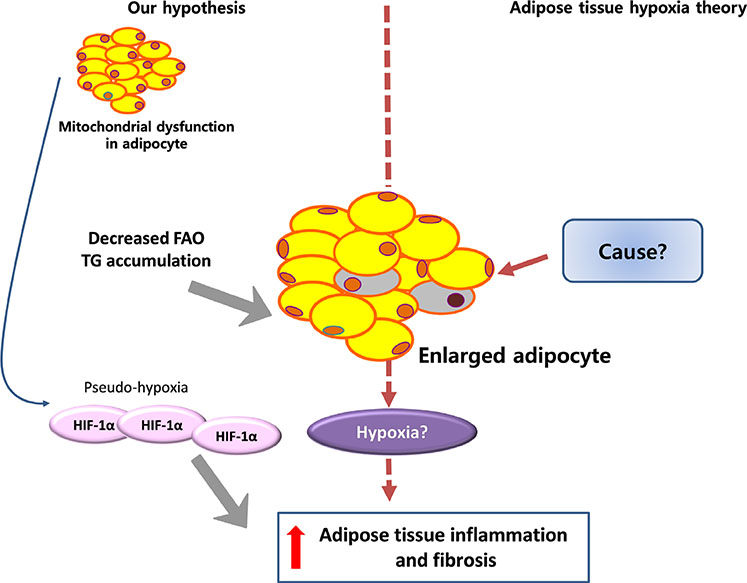
- Adipose tissue inflammation is considered a major contributing factor in the development of obesity-associated insulin resistance and cardiovascular diseases. However, the cause of adipose tissue inflammation is presently unclear. The role of mitochondria in white adipocytes has long been neglected because of their low abundance. However, recent evidence suggests that mitochondria are essential for maintaining metabolic homeostasis in white adipocytes. In a series of recent studies, we found that mitochondrial function in white adipocytes is essential to the synthesis of adiponectin, which is the most abundant adipokine synthesized from adipocytes, with many favorable effects on metabolism, including improvement of insulin sensitivity and reduction of atherosclerotic processes and systemic inflammation. From these results, we propose a new hypothesis that mitochondrial dysfunction in adipocytes is a primary cause of adipose tissue inflammation and compared this hypothesis with a prevailing concept that "adipose tissue hypoxia" may underlie adipose tissue dysfunction in obesity. Recent studies have emphasized the role of the mitochondrial quality control mechanism in maintaining mitochondrial function. Future studies are warranted to test whether an inadequate mitochondrial quality control mechanism is responsible for mitochondrial dysfunction in adipocytes and adipose tissue inflammation.
Keyword
MeSH Terms
Figure
Cited by 2 articles
-
Diabetes and Metabolism Journal in 2020: Good to Great
In-Kyung Jeong
Diabetes Metab J. 2020;44(1):1-2. doi: 10.4093/dmj.2020.0032.Impact of Skeletal Muscle Mass on Metabolic Health
Gyuri Kim, Jae Hyeon Kim
Endocrinol Metab. 2020;35(1):1-6. doi: 10.3803/EnM.2020.35.1.1.
Reference
-
1. Lackey DE, Olefsky JM. Regulation of metabolism by the innate immune system. Nat Rev Endocrinol. 2016; 12:15–28.2. Cani PD, Amar J, Iglesias MA, Poggi M, Knauf C, Bastelica D, Neyrinck AM, Fava F, Tuohy KM, Chabo C, Waget A, Delmee E, Cousin B, Sulpice T, Chamontin B, Ferrieres J, Tanti JF, Gibson GR, Casteilla L, Delzenne NM, Alessi MC, Burcelin R. Metabolic endotoxemia initiates obesity and insulin resistance. Diabetes. 2007; 56:1761–1772.3. Luck H, Tsai S, Chung J, Clemente-Casares X, Ghazarian M, Revelo XS, Lei H, Luk CT, Shi SY, Surendra A, Copeland JK, Ahn J, Prescott D, Rasmussen BA, Chng MH, Engleman EG, Girardin SE, Lam TK, Croitoru K, Dunn S, Philpott DJ, Guttman DS, Woo M, Winer S, Winer DA. Regulation of obesity-related insulin resistance with gut anti-inflammatory agents. Cell Metab. 2015; 21:527–542.4. Murano I, Barbatelli G, Parisani V, Latini C, Muzzonigro G, Castellucci M, Cinti S. Dead adipocytes, detected as crown-like structures, are prevalent in visceral fat depots of genetically obese mice. J Lipid Res. 2008; 49:1562–1568.5. Cinti S, Mitchell G, Barbatelli G, Murano I, Ceresi E, Faloia E, Wang S, Fortier M, Greenberg AS, Obin MS. Adipocyte death defines macrophage localization and function in adipose tissue of obese mice and humans. J Lipid Res. 2005; 46:2347–2355.6. Kwak SH, Park KS, Lee KU, Lee HK. Mitochondrial metabolism and diabetes. J Diabetes Investig. 2010; 1:161–169.7. Nunnari J, Suomalainen A. Mitochondria: in sickness and in health. Cell. 2012; 148:1145–1159.8. Hock MB, Kralli A. Transcriptional control of mitochondrial biogenesis and function. Annu Rev Physiol. 2009; 71:177–203.9. Kim MJ, Koo YD, Kim M, Lim S, Park YJ, Chung SS, Jang HC, Park KS. Rg3 improves mitochondrial function and the expression of key genes involved in mitochondrial biogenesis in C2C12 myotubes. Diabetes Metab J. 2016; 40:406–413.10. Kusminski CM, Scherer PE. Mitochondrial dysfunction in white adipose tissue. Trends Endocrinol Metab. 2012; 23:435–443.11. Flachs P, Rossmeisl M, Kuda O, Kopecky J. Stimulation of mitochondrial oxidative capacity in white fat independent of UCP1: a key to lean phenotype. Biochim Biophys Acta. 2013; 1831:986–1003.12. Boudina S, Graham TE. Mitochondrial function/dysfunction in white adipose tissue. Exp Physiol. 2014; 99:1168–1178.13. Koh EH, Park JY, Park HS, Jeon MJ, Ryu JW, Kim M, Kim SY, Kim MS, Kim SW, Park IS, Youn JH, Lee KU. Essential role of mitochondrial function in adiponectin synthesis in adipocytes. Diabetes. 2007; 56:2973–2981.14. Koh EH, Kim M, Ranjan KC, Kim HS, Park HS, Oh KS, Park IS, Lee WJ, Kim MS, Park JY, Youn JH, Lee KU. eNOS plays a major role in adiponectin synthesis in adipocytes. Am J Physiol Endocrinol Metab. 2010; 298:E846–E853.15. Koh EH, Kim AR, Kim H, Kim JH, Park HS, Ko MS, Kim MO, Kim HJ, Kim BJ, Yoo HJ, Kim SJ, Oh JS, Woo CY, Jang JE, Leem J, Cho MH, Lee KU. 11β-HSD1 reduces metabolic efficacy and adiponectin synthesis in hypertrophic adipocytes. J Endocrinol. 2015; 225:147–158.16. Hosogai N, Fukuhara A, Oshima K, Miyata Y, Tanaka S, Segawa K, Furukawa S, Tochino Y, Komuro R, Matsuda M, Shimomura I. Adipose tissue hypoxia in obesity and its impact on adipocytokine dysregulation. Diabetes. 2007; 56:901–911.17. Sun K, Tordjman J, Clement K, Scherer PE. Fibrosis and adipose tissue dysfunction. Cell Metab. 2013; 18:470–477.18. Trayhurn P. Hypoxia and adipose tissue function and dysfunction in obesity. Physiol Rev. 2013; 93:1–21.19. Kadowaki T, Yamauchi T, Kubota N, Hara K, Ueki K, Tobe K. Adiponectin and adiponectin receptors in insulin resistance, diabetes, and the metabolic syndrome. J Clin Invest. 2006; 116:1784–1792.20. Hoffstedt J, Arvidsson E, Sjolin E, Wahlen K, Arner P. Adipose tissue adiponectin production and adiponectin serum concentration in human obesity and insulin resistance. J Clin Endocrinol Metab. 2004; 89:1391–1396.21. Yu YH, Zhu H. Chronological changes in metabolism and functions of cultured adipocytes: a hypothesis for cell aging in mature adipocytes. Am J Physiol Endocrinol Metab. 2004; 286:E402–E410.22. Wilson-Fritch L, Burkart A, Bell G, Mendelson K, Leszyk J, Nicoloro S, Czech M, Corvera S. Mitochondrial biogenesis and remodeling during adipogenesis and in response to the insulin sensitizer rosiglitazone. Mol Cell Biol. 2003; 23:1085–1094.23. Olefsky JM, Saltiel AR. PPAR gamma and the treatment of insulin resistance. Trends Endocrinol Metab. 2000; 11:362–368.24. Maeda N, Takahashi M, Funahashi T, Kihara S, Nishizawa H, Kishida K, Nagaretani H, Matsuda M, Komuro R, Ouchi N, Kuriyama H, Hotta K, Nakamura T, Shimomura I, Matsuzawa Y. PPARgamma ligands increase expression and plasma concentrations of adiponectin, an adipose-derived protein. Diabetes. 2001; 50:2094–2099.25. Choo HJ, Kim JH, Kwon OB, Lee CS, Mun JY, Han SS, Yoon YS, Yoon G, Choi KM, Ko YG. Mitochondria are impaired in the adipocytes of type 2 diabetic mice. Diabetologia. 2006; 49:784–791.26. Villena JA. New insights into PGC-1 coactivators: redefining their role in the regulation of mitochondrial function and beyond. FEBS J. 2015; 282:647–672.27. Duplain H, Burcelin R, Sartori C, Cook S, Egli M, Lepori M, Vollenweider P, Pedrazzini T, Nicod P, Thorens B, Scherrer U. Insulin resistance, hyperlipidemia, and hypertension in mice lacking endothelial nitric oxide synthase. Circulation. 2001; 104:342–345.28. Lundberg JO, Gladwin MT, Weitzberg E. Strategies to increase nitric oxide signalling in cardiovascular disease. Nat Rev Drug Discov. 2015; 14:623–641.29. Nisoli E, Clementi E, Paolucci C, Cozzi V, Tonello C, Sciorati C, Bracale R, Valerio A, Francolini M, Moncada S, Carruba MO. Mitochondrial biogenesis in mammals: the role of endogenous nitric oxide. Science. 2003; 299:896–899.30. Kumari M, Chandola T, Brunner E, Kivimaki M. A nonlinear relationship of generalized and central obesity with diurnal cortisol secretion in the Whitehall II study. J Clin Endocrinol Metab. 2010; 95:4415–4423.31. Hackett RA, Steptoe A, Kumari M. Association of diurnal patterns in salivary cortisol with type 2 diabetes in the Whitehall II study. J Clin Endocrinol Metab. 2014; 99:4625–4631.32. Dube S, Norby BJ, Pattan V, Carter RE, Basu A, Basu R. 11β-Hydroxysteroid dehydrogenase types 1 and 2 activity in subcutaneous adipose tissue in humans: implications in obesity and diabetes. J Clin Endocrinol Metab. 2015; 100:E70–E76.33. Livingstone DE, Kenyon CJ, Walker BR. Mechanisms of dysregulation of 11 beta-hydroxysteroid dehydrogenase type 1 in obese Zucker rats. J Endocrinol. 2000; 167:533–539.34. Masuzaki H, Paterson J, Shinyama H, Morton NM, Mullins JJ, Seckl JR, Flier JS. A transgenic model of visceral obesity and the metabolic syndrome. Science. 2001; 294:2166–2170.35. Qatanani M, Tan Y, Dobrin R, Greenawalt DM, Hu G, Zhao W, Olefsky JM, Sears DD, Kaplan LM, Kemp DM. Inverse regulation of inflammation and mitochondrial function in adipose tissue defines extreme insulin sensitivity in morbidly obese patients. Diabetes. 2013; 62:855–863.36. Ryu MJ, Kim SJ, Kim YK, Choi MJ, Tadi S, Lee MH, Lee SE, Chung HK, Jung SB, Kim HJ, Jo YS, Kim KS, Lee SH, Kim JM, Kweon GR, Park KC, Lee JU, Kong YY, Lee CH, Chung J, Shong M. Crif1 deficiency reduces adipose OXPHOS capacity and triggers inflammation and insulin resistance in mice. PLoS Genet. 2013; 9:e1003356.37. Vernochet C, Damilano F, Mourier A, Bezy O, Mori MA, Smyth G, Rosenzweig A, Larsson NG, Kahn CR. Adipose tissue mitochondrial dysfunction triggers a lipodystrophic syndrome with insulin resistance, hepatosteatosis, and cardiovascular complications. FASEB J. 2014; 28:4408–4419.38. Heinonen S, Buzkova J, Muniandy M, Kaksonen R, Ollikainen M, Ismail K, Hakkarainen A, Lundbom J, Lundbom N, Vuolteenaho K, Moilanen E, Kaprio J, Rissanen A, Suomalainen A, Pietilainen KH. Impaired mitochondrial biogenesis in adipose tissue in acquired obesity. Diabetes. 2015; 64:3135–3145.39. Jang JY, Blum A, Liu J, Finkel T. The role of mitochondria in aging. J Clin Invest. 2018; 128:3662–3670.40. Sun N, Youle RJ, Finkel T. The mitochondrial basis of aging. Mol Cell. 2016; 61:654–666.41. Soro-Arnaiz I, Li QOY, Torres-Capelli M, Melendez-Rodriguez F, Veiga S, Veys K, Sebastian D, Elorza A, Tello D, Hernansanz-Agustin P, Cogliati S, Moreno-Navarrete JM, Balsa E, Fuertes E, Romanos E, Martinez-Ruiz A, Enriquez JA, Fernandez-Real JM, Zorzano A, De Bock K, Aragones J. Role of mitochondrial complex IV in age-dependent obesity. Cell Rep. 2016; 16:2991–3002.42. Adamczak M, Rzepka E, Chudek J, Wiecek A. Ageing and plasma adiponectin concentration in apparently healthy males and females. Clin Endocrinol (Oxf). 2005; 62:114–118.43. Miles EA, Rees D, Banerjee T, Cazzola R, Lewis S, Wood R, Oates R, Tallant A, Cestaro B, Yaqoob P, Wahle KW, Calder PC. Age-related increases in circulating inflammatory markers in men are independent of BMI, blood pressure and blood lipid concentrations. Atherosclerosis. 2008; 196:298–305.44. Guzik TJ, Skiba DS, Touyz RM, Harrison DG. The role of infiltrating immune cells in dysfunctional adipose tissue. Cardiovasc Res. 2017; 113:1009–1023.45. Jang JE, Ko MS, Yun JY, Kim MO, Kim JH, Park HS, Kim AR, Kim HJ, Kim BJ, Ahn YE, Oh JS, Lee WJ, Harris RA, Koh EH, Lee KU. Nitric oxide produced by macrophages inhibits adipocyte differentiation and promotes profibrogenic responses in preadipocytes to induce adipose tissue fibrosis. Diabetes. 2016; 65:2516–2528.46. Strissel KJ, Stancheva Z, Miyoshi H, Perfield JW 2nd, DeFuria J, Jick Z, Greenberg AS, Obin MS. Adipocyte death, adipose tissue remodeling, and obesity complications. Diabetes. 2007; 56:2910–2918.47. Galluzzi L, Kepp O, Kroemer G. Mitochondria: master regulators of danger signalling. Nat Rev Mol Cell Biol. 2012; 13:780–788.48. Green DR, Galluzzi L, Kroemer G. Cell biology. Metabolic control of cell death. Science. 2014; 345:1250256.49. Omar A, Chatterjee TK, Tang Y, Hui DY, Weintraub NL. Proinflammatory phenotype of perivascular adipocytes. Arterioscler Thromb Vasc Biol. 2014; 34:1631–1636.50. Kanda H, Tateya S, Tamori Y, Kotani K, Hiasa K, Kitazawa R, Kitazawa S, Miyachi H, Maeda S, Egashira K, Kasuga M. MCP-1 contributes to macrophage infiltration into adipose tissue, insulin resistance, and hepatic steatosis in obesity. J Clin Invest. 2006; 116:1494–1505.51. Zhao H, Liu YJ, Liu ZR, Tang DD, Chen XW, Chen YH, Zhou RN, Chen SQ, Niu HX. Role of mitochondrial dysfunction in renal fibrosis promoted by hypochlorite-modified albumin in a remnant kidney model and protective effects of antioxidant peptide SS-31. Eur J Pharmacol. 2017; 804:57–67.52. Long YC, Zierath JR. AMP-activated protein kinase signaling in metabolic regulation. J Clin Invest. 2006; 116:1776–1783.53. Kim MJ, Kim EH, Pun NT, Chang JH, Kim JA, Jeong JH, Choi DY, Kim SH, Park PH. Globular adiponectin inhibits lipopolysaccharide-primed inflammasomes activation in macrophages via autophagy induction: the critical role of AMPK signaling. Int J Mol Sci. 2017; 18:E1275.54. Griffiths HR, Gao D, Pararasa C. Redox regulation in metabolic programming and inflammation. Redox Biol. 2017; 12:50–57.55. Chan KL, Pillon NJ, Sivaloganathan DM, Costford SR, Liu Z, Theret M, Chazaud B, Klip A. Palmitoleate reverses high fat-induced proinflammatory macrophage polarization via AMP-activated protein kinase (AMPK). J Biol Chem. 2015; 290:16979–16988.56. Lovren F, Pan Y, Quan A, Szmitko PE, Singh KK, Shukla PC, Gupta M, Chan L, Al-Omran M, Teoh H, Verma S. Adiponectin primes human monocytes into alternative anti-inflammatory M2 macrophages. Am J Physiol Heart Circ Physiol. 2010; 299:H656–H663.57. Hahn WS, Kuzmicic J, Burrill JS, Donoghue MA, Foncea R, Jensen MD, Lavandero S, Arriaga EA, Bernlohr DA. Proinflammatory cytokines differentially regulate adipocyte mitochondrial metabolism, oxidative stress, and dynamics. Am J Physiol Endocrinol Metab. 2014; 306:E1033–E1045.58. Pickles S, Vigie P, Youle RJ. Mitophagy and quality control mechanisms in mitochondrial maintenance. Curr Biol. 2018; 28:R170–R185.59. Green DR, Galluzzi L, Kroemer G. Mitochondria and the autophagy-inflammation-cell death axis in organismal aging. Science. 2011; 333:1109–1112.60. Novak I. Mitophagy: a complex mechanism of mitochondrial removal. Antioxid Redox Signal. 2012; 17:794–802.61. Cui C, Chen S, Qiao J, Qing L, Wang L, He T, Wang C, Liu F, Gong L, Chen L, Hou X. PINK1-Parkin alleviates metabolic stress induced by obesity in adipose tissue and in 3T3-L1 preadipocytes. Biochem Biophys Res Commun. 2018; 498:445–452.62. Heo JW, No MH, Park DH, Kang JH, Seo DY, Han J, Neufer PD, Kwak HB. Effects of exercise on obesity-induced mitochondrial dysfunction in skeletal muscle. Korean J Physiol Pharmacol. 2017; 21:567–577.63. Trevellin E, Scorzeto M, Olivieri M, Granzotto M, Valerio A, Tedesco L, Fabris R, Serra R, Quarta M, Reggiani C, Nisoli E, Vettor R. Exercise training induces mitochondrial biogenesis and glucose uptake in subcutaneous adipose tissue through eNOS-dependent mechanisms. Diabetes. 2014; 63:2800–2811.64. Stanford KI, Middelbeek RJ, Goodyear LJ. Exercise effects on white adipose tissue: beiging and metabolic adaptations. Diabetes. 2015; 64:2361–2368.65. Bogacka I, Xie H, Bray GA, Smith SR. Pioglitazone induces mitochondrial biogenesis in human subcutaneous adipose tissue in vivo. Diabetes. 2005; 54:1392–1399.66. Teixeira J, Chavarria D, Borges F, Wojtczak L, Wieckowski MR, Karkucinska-Wieckowska A, Oliveira PJ. Dietary polyphenols and mitochondrial function: role in health and disease. Curr Med Chem. 2017; 05. 28. DOI: 10.2174/0929867324666170529101810. [Epub].67. Ye J, Gao Z, Yin J, He Q. Hypoxia is a potential risk factor for chronic inflammation and adiponectin reduction in adipose tissue of ob/ob and dietary obese mice. Am J Physiol Endocrinol Metab. 2007; 293:E1118–E1128.68. McLaughlin T, Ackerman SE, Shen L, Engleman E. Role of innate and adaptive immunity in obesity-associated metabolic disease. J Clin Invest. 2017; 127:5–13.69. Rasouli N. Adipose tissue hypoxia and insulin resistance. J Investig Med. 2016; 64:830–832.70. Trayhurn P, Wang B, Wood IS. Hypoxia in adipose tissue: a basis for the dysregulation of tissue function in obesity? Br J Nutr. 2008; 100:227–235.71. Rausch ME, Weisberg S, Vardhana P, Tortoriello DV. Obesity in C57BL/6J mice is characterized by adipose tissue hypoxia and cytotoxic T-cell infiltration. Int J Obes (Lond). 2008; 32:451–463.72. Goossens GH, Bizzarri A, Venteclef N, Essers Y, Cleutjens JP, Konings E, Jocken JW, Cajlakovic M, Ribitsch V, Clement K, Blaak EE. Increased adipose tissue oxygen tension in obese compared with lean men is accompanied by insulin resistance, impaired adipose tissue capillarization, and inflammation. Circulation. 2011; 124:67–76.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Macrophage and Adipocyte Mitochondrial Dysfunction in Obesity-Induced Metabolic Diseases
- The Emerging Importance of Mitochondria in White Adipocytes: Neither Last nor Least
- Cellular and Intercellular Homeostasis in Adipose Tissue with Mitochondria-Specific Stress
- Eosinophils and Type 2 Cytokine Signaling in Macrophages Support the Biogenesis of Cold-induced Beige Fat
- The Role of Vitamin D in Adipose Tissue Biology: Adipocyte Differentiation, Energy Metabolism, and Inflammation