J Korean Soc Radiol.
2019 May;80(3):524-536. 10.3348/jksr.2019.80.3.524.
Usefulness of Apparent Diffusion Coefficient Value of Diffusion-Weighted Imaging and Peak Standardized Uptake Values of Positron Emission Tomography-CT for Predicting Prognostic Factors of Breast Cancer
- Affiliations
-
- 1Department of Radiology, Konyang University Hospital, Daejeon, Korea. radkim14@gmail.com
- 2Department of Preventive Medicine, Konyang University Hospital, Daejeon, Korea.
- KMID: 2454031
- DOI: http://doi.org/10.3348/jksr.2019.80.3.524
Abstract
- PURPOSE
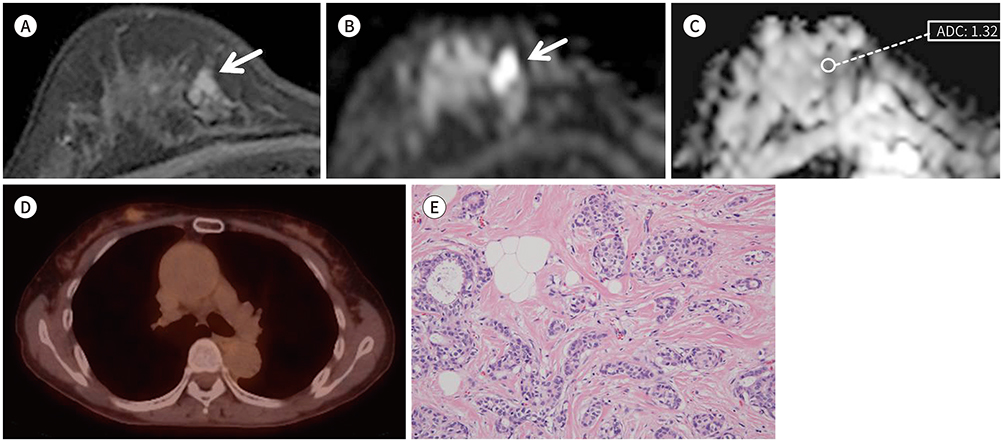
This study was performed to retrospectively correlate the apparent diffusion coefficient (ADC) value and peak standardized uptake value (pSUV) with prognostic factors and MRI findings for breast lesions.
MATERIALS AND METHODS
Ninety four breast cancers in 82 women were included in this study. Our patients underwent presurgical MRI including diffusion-weighted imaging (DWI), 18-fluorodeoxyglucose PET-CT, and immunohistological staining of the surgical or biopsy specimens. We evaluated relationships between mean ADCs and pSUVs with a variety of prognostic factors (age, tumor size, histologic grade of tumor, hormone receptors, human epidermal growth factor receptor 2 expression status, and nodal metastasis) and MRI findings (shape, margin and internal enhancement of mass, T2-signal intensity, and kinetics), using statistical methods.
RESULTS
Both mean ADCs and pSUVs were significantly associated with histologic grade (p = 0.000 and p = 0.001) and nodal metastasis (p = 0.013 and p = 0.001). pSUVs were significantly associated with tumor size and estrogen receptor status, as well as irregular shape and rim enhancement pattern on MRI findings. On multivariate analysis, mean ADCs were significantly associated with invasiveness, estrogen receptor status and HER-2 expression status. PSUVs were only significantly associated with tumor size.
CONCLUSION
Mean pSUVs on PET-CT and ADCs on DWI helped predict prognosis of breast cancer.
MeSH Terms
Figure
Reference
-
1. DeSantis CE, Bray F, Ferlay J, Lortet-Tieulent J, Anderson BO, Jemal A. International variation in female breast cancer incidence and mortality rates. Cancer Epidemiol Biomarkers Prev. 2015; 24:1495–1506.
Article2. Harding C, Pompei F, Burmistrov D, Welch HG, Abebe R, Wilson R. Breast cancer screening, incidence, and mortality across US counties. JAMA Intern Med. 2015; 175:1483–1489.
Article3. Li J, Chen Z, Su K, Zeng J. Clinicopathological classification and traditional prognostic indicators of breast cancer. Int J Clin Exp Pathol. 2015; 8:8500–8505.4. Arul P, Masilamani S. Comparative evaluation of various cytomorphological grading systems in breast carcinoma. Indian J Med Paediatr Oncol. 2016; 37:79–84.
Article5. Runowicz CD, Leach CR, Henry NL, Henry KS, Mackey HT, Cowens-Alvarado RL, et al. American Cancer Society/American Society of Clinical Oncology breast cancer survivorship care guideline. CA Cancer J Clin. 2016; 66:43–73.
Article6. Baltzer PA, Benndorf M, Dietzel M, Gajda M, Runnebaum IB, Kaiser WA. False-positive findings at contrast-enhanced breast MRI: a BI-RADS descriptor study. AJR Am J Roentgenol. 2010; 194:1658–1663.
Article7. Heusner TA, Kuemmel S, Koeninger A, Hamami ME, Hahn S, Quinsten A, et al. Diagnostic value of diffusion-weighted magnetic resonance imaging (DWI) compared to FDG PET/CT for whole-body breast cancer staging. Eur J Nucl Med Mol Imaging. 2010; 37:1077–1086.
Article8. Kızıldağ Yırgın İ, Arslan G, Öztürk E, Yırgın H, Taşdemir N, Gemici AA, et al. Diffusion weighted MR imaging of breast and correlation of prognostic factors in breast cancer. Balkan Med J. 2016; 33:301–307.
Article9. Razek AA, Gaballa G, Denewer A, Nada N. Invasive ductal carcinoma: correlation of apparent diffusion coefficient value with pathological prognostic factors. NMR Biomed. 2010; 23:619–623.
Article10. Kitajima K, Yamano T, Fukushima K, Miyoshi Y, Hirota S, Kawanaka Y, et al. Correlation of the SUVmax of FDG-PET and ADC values of diffusion-weighted MR imaging with pathologic prognostic factors in breast carcinoma. Eur J Radiol. 2016; 85:943–949.
Article11. Choi BB, Kim SH, Kang BJ, Lee , JH , Song BJ, Jeong SH, et al. Diffusion-weighted imaging and FDG PET/CT: predicting the prognoses with apparent diffusion coefficient values and maximum standardized uptake values in patients with invasive ductal carcinoma. World J Surg Oncol. 2012; 10:126.
Article12. Karan B, Pourbagher A, Torun N. Diffusion-weighted imaging and 18F-fluorodeoxyglucose positron emission tomography/computed tomography in breast cancer: correlation of the apparent diffusion coefficient and maximum standardized uptake values with prognostic factors. J Magn Reson Imaging. 2016; 43:1434–1444.13. Park SH, Choi HY, Hahn SY. Correlations between apparent diffusion coefficient values of invasive ductal carcinoma and pathologic factors on diffusion-weighted MRI at 3.0 Tesla. J Magn Reson Imaging. 2015; 41:175–182.
Article14. Krammer J, Schnitzer A, Kaiser CG, Buesing KA, Sperk E, Brade J, et al. 18F-FDG PET/CT for initial staging in breast cancer patients - Is there a relevant impact on treatment planning compared to conventional staging modalities. Eur Radiol. 2015; 25:2460–2469.15. Jadvar H, Alavi A, Gambhir SS. 18F-FDG uptake in lung, breast, and colon cancers: molecular biology correlates and disease characterization. J Nucl Med. 2009; 50:1820–1827.16. Nakajo M, Kajiya Y, Kaneko T, Kaneko Y, Takasaki T, Tani A, et al. FDG PET/CT and diffusion-weighted imaging for breast cancer: prognostic value of maximum standardized uptake values and apparent diffusion coefficient values of the primary lesion. Eur J Nucl Med Mol Imaging. 2010; 37:2011–2020.
Article17. Baba S, Isoda T, Maruoka Y, Kitamura Y, Sasaki M, Yoshida T, et al. Diagnostic and prognostic value of pretreatment SUV in 18F-FDG/PET in breast cancer: comparison with apparent diffusion coefficient from diffusion-weighted MR imaging. J Nucl Med. 2014; 55:736–742.18. Fan YS, Casas CE, Peng J, Watkins M, Fan L, Chapman J, et al. HER2 FISH classification of equivocal HER2 IHC breast cancers with use of the 2013 ASCO/CAP practice guideline. Breast Cancer Res Treat. 2016; 155:457–462.
Article19. Orel SG. High-resolution MR imaging of the breast. Semin Ultrasound CT MR. 1996; 17:476–493.
Article20. Kuhl CK. MRI of breast tumors. Eur Radiol. 2000; 10:46–58.
Article21. Bartella L, Smith CS, Dershaw DD, Liberman L. Imaging breast cancer. Radiol Clin North Am. 2007; 45:45–67.
Article22. Oshida M, Uno K, Suzuki M, Nagashima T, Hashimoto H, Yagata H, et al. Predicting the prognoses of breast carcinoma patients with positron emission tomography using 2-deoxy-2-fluoro[18F]-D-glucose. Cancer. 1998; 82:2227–2234.
Article23. Kitajima K, Miyoshi Y, Yamano T, Odawara S, Higuchi T, Yamakado K. Prognostic value of FDG-PET and DWI in breast cancer. Ann Nucl Med. 2018; 32:44–53.
Article24. Elston CW, Ellis IO. Pathological prognostic factors in breast cancer. I. The value of histological grade in breast cancer: experience from a large study with long-term follow-up. Histopathology. 1991; 19:403–410.
Article25. Elston CW, Ellis IO, Pinder SE. Pathological prognostic factors in breast cancer. Crit Rev Oncol Hematol. 1999; 31:209–223.
Article26. Park EK, Cho KR, Seo BK, Woo OH, Cho SB, Bae JW. Additional value of diffusion-weighted imaging to evaluate prognostic factors of breast cancer: correlation with the apparent diffusion coefficient. Iran J Radiol. 2016; 13:e33133.
Article27. Hatakenaka M, Soeda H, Yabuuchi H, Matsuo Y, Kamitani T, Oda Y, et al. Apparent diffusion coefficients of breast tumors: clinical application. Magn Reson Med Sci. 2008; 7:23–29.
Article28. Ulaner GA, Eaton A, Morris PG, Lilienstein J, Jhaveri K, Patil S, et al. Prognostic value of quantitative fluorodeoxyglucose measurements in newly diagnosed metastatic breast cancer. Cancer Med. 2013; 2:725–733.
Article29. Yoshikawa MI, Ohsumi S, Sugata S, Kataoka M, Takashima S, Mochizuki T, et al. Relation between cancer cellularity and apparent diffusion coefficient values using diffusion-weighted magnetic resonance imaging in breast cancer. Radiat Med. 2008; 26:222–226.
Article30. Rigo P, Paulus P, Kaschten BJ, Hustinx R, Bury T, Jerusalem G, et al. Oncological applications of positron emission tomography with fluorine-18 fluorodeoxyglucose. Eur J Nucl Med. 1996; 23:1641–1674.
Article31. Kumar R, Chauhan A, Zhuang H, Chandra P, Schnall M, Alavi A. Clinicopathologic factors associated with false negative FDG-PET in primary breast cancer. Breast Cancer Res Treat. 2006; 98:267–274.
Article32. Ludovini V, Sidoni A, Pistola L, Bellezza G, De Angelis V, Gori S, et al. Evaluation of the prognostic role of vascular endothelial growth factor and microvessel density in stages I and II breast cancer patients. Breast Cancer Res Treat. 2003; 81:159–168.
Article33. Chung J, Youk JH, Kim JA, Gweon HM, Kim EK, Ryu YH, et al. Role of diffusion-weighted MRI: predicting axillary lymph node metastases in breast cancer. Acta Radiol. 2014; 55:909–916.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- The Predictability for the Prognosis of Breast Cancer Using the Apparent Diffusion Coefficient Value of Diffusion-Weighted 3 T MRI and the Standardized Uptake Value of Positron Emission Tomography/CT: Assessment of Prognostic Factor
- Correlation of Prognostic Factors of Invasive Lobular Carcinoma with ADC Value of DWI and SUVMax of FDG-PET
- Reversal of a Large Ischemic Lesion with Low Apparent Diffusion Coefficient Value by Rapid Spontaneous Recanalization
- Diffusion-Weighted MRI for the Initial Viability Evaluation of Parasites in Hepatic Alveolar Echinococcosis: Comparison with Positron Emission Tomography
- Correlation Between Apparent Diffusion Coefficients and Standardized Uptake Values in Hybrid 18F-FDG PET/MR: Preliminary Results in Rectal Cancer