Yonsei Med J.
2012 Sep;53(5):1036-1044.
Gene Expression Pattern of Human Chorion-Derived Mesenchymal Stem Cells during Adipogenic Differentiation
- Affiliations
-
- 1Department of Obstetrics and Gynecology, Uijeongbu St. Mary's Hospital, School of Medicine, The Catholic University of Korea, Uijeongbu, Korea.
- 2Department of Obstetrics and Gynecology, St. Vincent's Hospital, School of Medicine, The Catholic University of Korea, Suwon, Korea.
- 3Department of Obstetrics and Gynecology, Seoul St. Mary's Hospital, School of Medicine, The Catholic University of Korea, Seoul, Korea. jcshin@catholic.ac.kr
Abstract
- PURPOSE
The aim of this study was to identify the adipocyte-specific gene expression patterns in chorion-derived mesenchymal stem cells during adipogenic differentiation.
MATERIALS AND METHODS
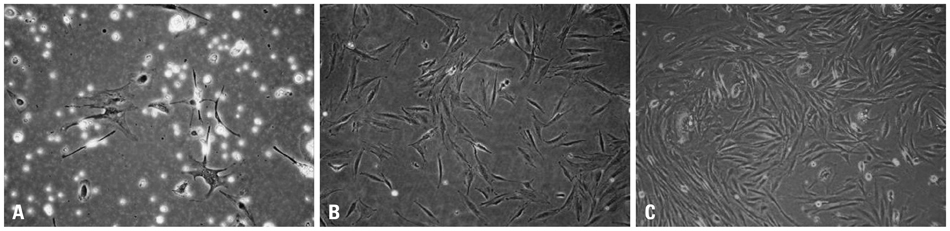
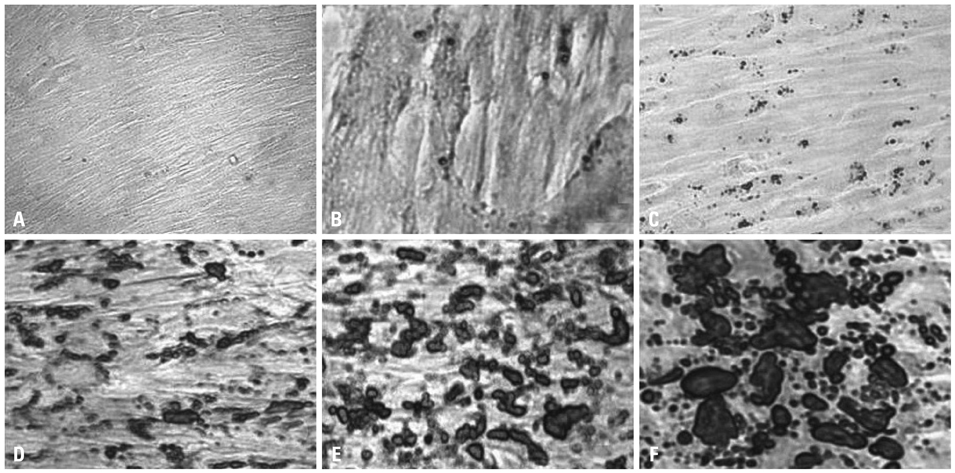
Chorionic cells were isolated from the third trimester chorions from human placenta at birth and identified morphologically and by fluorescence-activated cell sorting analysis. After inducing adipogenic differentiation for 28 days, cells at days 3, 10, 21 and 28 were analyzed by Oil red O staining and RNA extraction in order to assess the expression levels of adipocyte marker genes, including CCAAT-enhancer binding protein alpha (C/EBPalpha), peroxisome proliferator-activated receptor gamma (PPARgamma), fatty acid binding protein 4 (FABP4) and Glycerol-3-phosphate dehydrogenase (GPD2). Cells not induced for differentiation were compared with the induced cells as a control group.
RESULTS
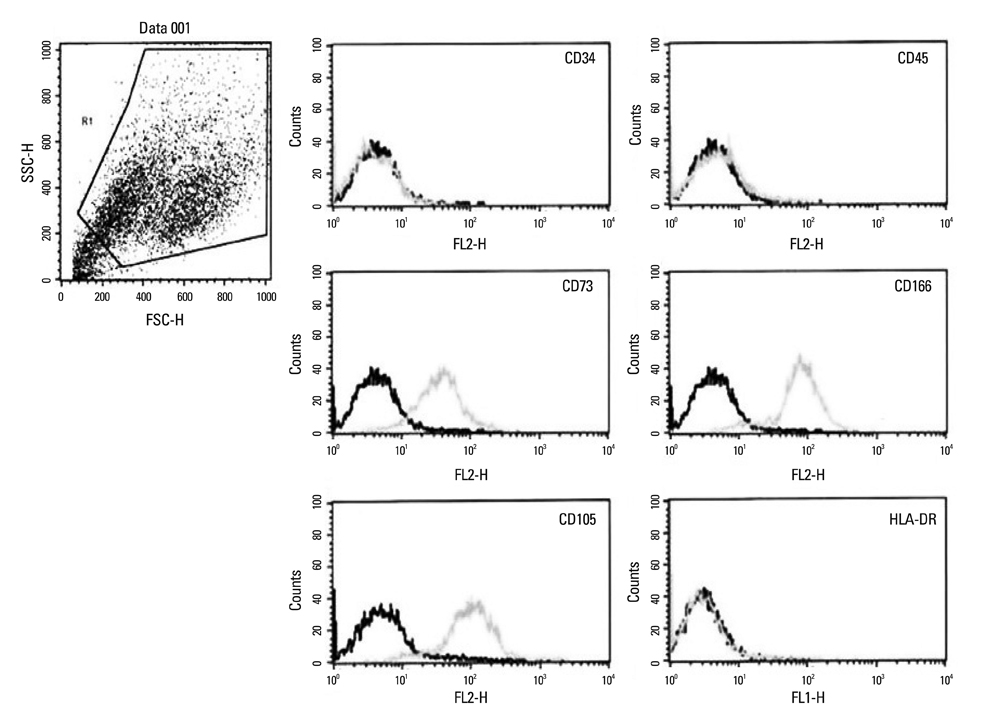
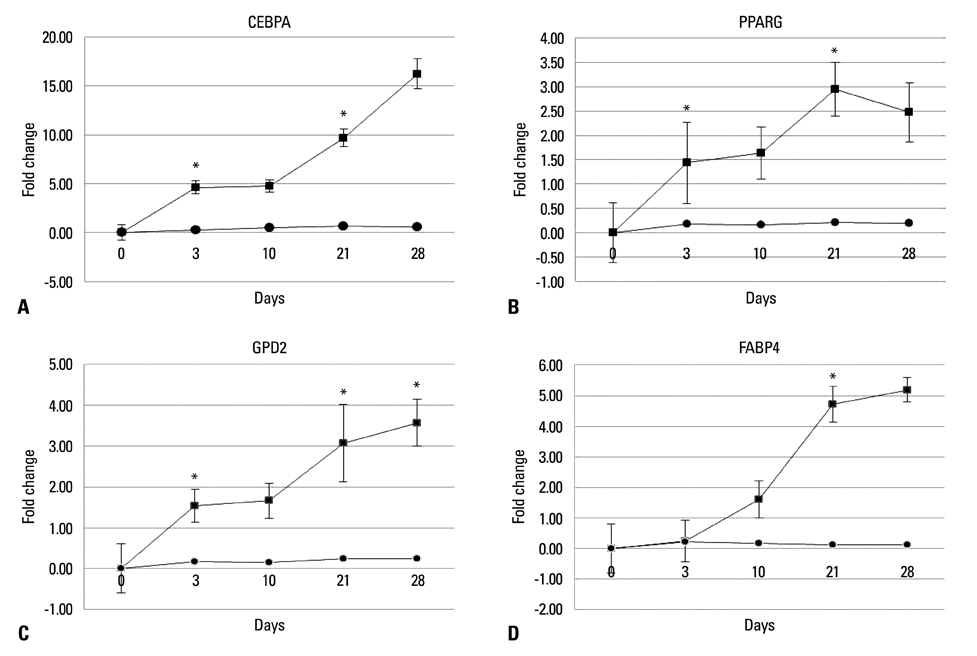
Chorion-derived cells showed the same pattern as fibroblasts, and expressed CD73, CD105, and CD166 antigens, but not CD45, CD34, and HLA-DR antigens. On day 3 after differentiation, cells began to stain positively upon Oil red O staining, and continuously increased in lipid granules for 4 weeks. The expression level of C/EBPalpha increased 4.6 fold on day 3 after induction, and continued to increase for 4 weeks. PPARgamma was expressed at a maximum of 2.9 fold on day 21. FABP4 and GPD2 were significantly expressed at 4.7- and 3.0-fold, respectively, on day 21, compared to controls, and further increased thereafter.
CONCLUSION
Human chorion-derived mesenchymal stem cells exhibited the sequential expression pattern of adipocyte marker genes during differentiation, corresponding to adipogenesis.
MeSH Terms
-
Activated-Leukocyte Cell Adhesion Molecule
Adipocytes
Adipogenesis
Carrier Proteins
Chorion
Female
Fibroblasts
Flow Cytometry
Gene Expression*
Glycerolphosphate Dehydrogenase
HLA-DR Antigens
Humans*
Mesenchymal Stromal Cells*
Parturition
Placenta
PPAR gamma
Pregnancy
Pregnancy Trimester, Third
RNA
Activated-Leukocyte Cell Adhesion Molecule
Carrier Proteins
Glycerolphosphate Dehydrogenase
HLA-DR Antigens
PPAR gamma
RNA
Figure
Reference
-
1. Pittenger MF, Mackay AM, Beck SC, Jaiswal RK, Douglas R, Mosca JD, et al. Multilineage potential of adult human mesenchymal stem cells. Science. 1999. 284:143–147.
Article2. Ferrari G, Cusella-De Angelis G, Coletta M, Paolucci E, Stornaiuolo A, Cossu G, et al. Muscle regeneration by bone marrow-derived myogenic progenitors. Science. 1998. 279:1528–1530.
Article3. Sanchez-Ramos J, Song S, Cardozo-Pelaez F, Hazzi C, Stedeford T, Willing A, et al. Adult bone marrow stromal cells differentiate into neural cells in vitro. Exp Neurol. 2000. 164:247–256.
Article4. Beyer Nardi N, da Silva Meirelles L. Mesenchymal stem cells: isolation, in vitro expansion and characterization. Handb Exp Pharmacol. 2006. 249–282.
Article5. Dvash T, Mayshar Y, Darr H, McElhaney M, Barker D, Yanuka O, et al. Temporal gene expression during differentiation of human embryonic stem cells and embryoid bodies. Hum Reprod. 2004. 19:2875–2883.
Article6. Yamamoto Y, Banas A, Murata S, Ishikawa M, Lim CR, Teratani T, et al. A comparative analysis of the transcriptome and signal pathways in hepatic differentiation of human adipose mesenchymal stem cells. FEBS J. 2008. 275:1260–1273.
Article7. Wang Q, Yu JH, Zhai HH, Zhao QT, Chen JW, Shu L, et al. Temporal expression of estrogen receptor alpha in rat bone marrow mesenchymal stem cells. Biochem Biophys Res Commun. 2006. 347:117–123.
Article8. Hu Y, Liao L, Wang Q, Ma L, Ma G, Jiang X, et al. Isolation and identification of mesenchymal stem cells from human fetal pancreas. J Lab Clin Med. 2003. 141:342–349.
Article9. in 't Anker PS, Noort WA, Scherjon SA, Kleijburg-van der Keur C, Kruisselbrink AB, van Bezooijen RL, et al. Mesenchymal stem cells in human second-trimester bone marrow, liver, lung, and spleen exhibit a similar immunophenotype but a heterogeneous multilineage differentiation potential. Haematologica. 2003. 88:845–852.10. Campagnoli C, Roberts IA, Kumar S, Bennett PR, Bellantuono I, Fisk NM. Identification of mesenchymal stem/progenitor cells in human first-trimester fetal blood, liver, and bone marrow. Blood. 2001. 98:2396–2402.
Article11. Battula VL, Bareiss PM, Treml S, Conrad S, Albert I, Hojak S, et al. Human placenta and bone marrow derived MSC cultured in serum-free, b-FGF-containing medium express cell surface frizzled-9 and SSEA-4 and give rise to multilineage differentiation. Differentiation. 2007. 75:279–291.
Article12. In't Anker PS, Scherjon SA, Kleijburg-van der, de Groot-Swings GM, Claas FH, Fibbe WE, et al. Isolation of mesenchymal stem cells of fetal or maternal origin from human placenta. Stem Cells. 2004. 22:1338–1345.13. Miki T, Lehmann T, Cai H, Stolz DB, Strom SC. Stem cell characteristics of amniotic epithelial cells. Stem Cells. 2005. 23:1549–1559.
Article14. Mitchell KE, Weiss ML, Mitchell BM, Martin P, Davis D, Morales L, et al. Matrix cells from Wharton's jelly form neurons and glia. Stem Cells. 2003. 21:50–60.
Article15. Portmann-Lanz CB, Schoeberlein A, Huber A, Sager R, Malek A, Holzgreve W, et al. Placental mesenchymal stem cells as potential autologous graft for pre- and perinatal neuroregeneration. Am J Obstet Gynecol. 2006. 194:664–673.
Article16. Otto TC, Lane MD. Adipose development: from stem cell to adipocyte. Crit Rev Biochem Mol Biol. 2005. 40:229–242.
Article17. Hutley LJ, Newell FM, Joyner JM, Suchting SJ, Herington AC, Cameron DP, et al. Effects of rosiglitazone and linoleic acid on human preadipocyte differentiation. Eur J Clin Invest. 2003. 33:574–581.
Article18. Soncini M, Vertua E, Gibelli L, Zorzi F, Denegri M, Albertini A, et al. Isolation and characterization of mesenchymal cells from human fetal membranes. J Tissue Eng Regen Med. 2007. 1:296–305.
Article19. Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) Method. Methods. 2001. 25:402–408.
Article20. Umek RM, Friedman AD, McKnight SL. CCAAT-enhancer binding protein: a component of a differentiation switch. Science. 1991. 251:288–292.
Article21. Tontonoz P, Hu E, Graves RA, Budavari AI, Spiegelman BM. mPPAR gamma 2: tissue-specific regulator of an adipocyte enhancer. Genes Dev. 1994. 8:1224–1234.
Article22. Rosen ED, Hsu CH, Wang X, Sakai S, Freeman MW, Gonzalez FJ, et al. C/EBPalpha induces adipogenesis through PPARgamma: a unified pathway. Genes Dev. 2002. 16:22–26.23. Shao D, Lazar MA. Peroxisome proliferator activated receptor gamma, CCAAT/enhancer-binding protein alpha, and cell cycle status regulate the commitment to adipocyte differentiation. J Biol Chem. 1997. 272:21473–21478.
Article24. Wu Z, Rosen ED, Brun R, Hauser S, Adelmant G, Troy AE, et al. Cross-regulation of C/EBP alpha and PPAR gamma controls the transcriptional pathway of adipogenesis and insulin sensitivity. Mol Cell. 1999. 3:151–158.
Article25. Auwerx J, Martin G, Guerre-Millo M, Staels B. Transcription, adipocyte differentiation, and obesity. J Mol Med (Berl). 1996. 74:347–352.
Article26. Shin DW, Kim SN, Lee SM, Lee W, Song MJ, Park SM, et al. (-)-Catechin promotes adipocyte differentiation in human bone marrow mesenchymal stem cells through PPAR gamma transactivation. Biochem Pharmacol. 2009. 77:125–133.
Article27. Chang YJ, Shih DT, Tseng CP, Hsieh TB, Lee DC, Hwang SM. Disparate mesenchyme-lineage tendencies in mesenchymal stem cells from human bone marrow and umbilical cord blood. Stem Cells. 2006. 24:679–685.
Article28. Karahuseyinoglu S, Kocaefe C, Balci D, Erdemli E, Can A. Functional structure of adipocytes differentiated from human umbilical cord stroma-derived stem cells. Stem Cells. 2008. 26:682–691.
Article29. Sekiya I, Larson BL, Vuoristo JT, Cui JG, Prockop DJ. Adipogenic differentiation of human adult stem cells from bone marrow stroma (MSCs). J Bone Miner Res. 2004. 19:256–264.
Article30. Kim HK, Im EH, Park H, Cho JA, Yang DY, Kim KH, et al. Characterization and differentiation into adipocytes of mesenchymal stem cells (MSCs) from human adipose tissue and amniotic fluid. Korean J Obstet Gynecol. 2009. 52:447–455.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Difference in Cell Characteristics among the Monoclonal Cell Populations Obtained from the Human Umbilical Cord Blood Derived Mesenchymal Stem Cell Population
- Dlx3 and Dlx5 Inhibit Adipogenic Differentiation of Human Dental Pulp Stem Cells
- Comparison with human amniotic membrane- and adipose tissue-derived mesenchymal stem cells
- Difference in HLA-DR Expression of Human Umbilical Cord Blood Derived Mesenchymal Stem Cells after Tri-lineage Differentiation
- Effects of nanoscale ridge/groovepattern arrayed surface on in vitro differentiation of multi-potent pulp cells derived from human supernumerary teeth