Yonsei Med J.
2012 Sep;53(5):968-973.
A Direct Inhibitory Effect of Botulinum Toxin Type A on Antral Circular Muscle Contractility of Guinea Pig
- Affiliations
-
- 1Department of Medicine, Kangbuk Samsung Hospital, Sungkyunkwan University School of Medicine, Seoul, Korea.
- 2Department of Medicine, Gangnam Severance Hospital, Yonsei University College of Medicine, Seoul, Korea. hjpark21@yuhs.ac
- 3Department of Physiology, Yonsei University College of Medicine, Seoul, Korea.
Abstract
- PURPOSE
Recent studies suggest new mechanisms of Botulinum toxin (BoNT) other than inhibiting acetylcholine (ACh) release from nerve terminals. The aim of this study was to determine whether other mechanisms for BoNT exist, so that it directly inhibits smooth muscle contraction.
MATERIALS AND METHODS
Guinea pig antral muscle strips were studied in vitro after 2 hours of exposure to Botulinum toxin type A (BoNT/A). Contractile responses to electric field stimulation (EFS), high K+ (60 mM) and ACh (100 microM) were evaluated 24 and 48 hours after antral intramuscular injection of BoNT/A or vehicle.
RESULTS
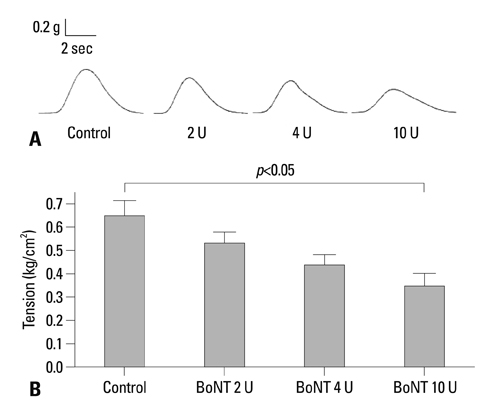
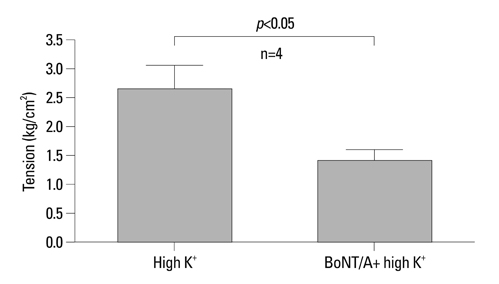
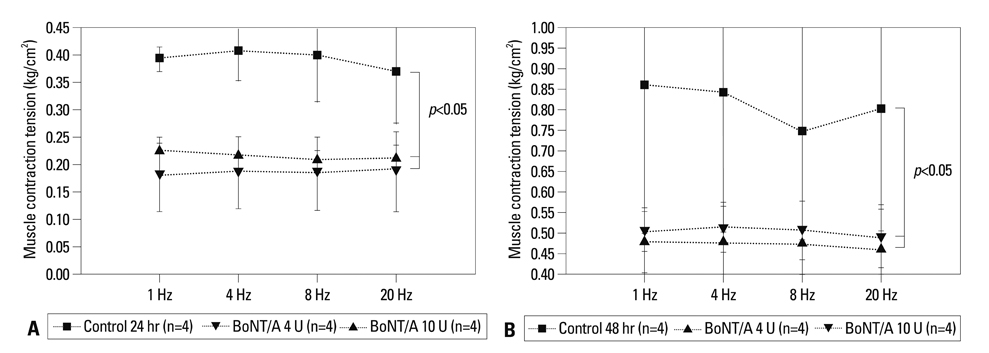
BoNT/A inhibited muscular contraction caused by high K+ and ACh. Contractile responses to low (1 & 4 Hz) and high (8 & 20 Hz) frequency EFS of antral muscle strips 24 and 48 hours after antral intramuscular injection of BoNT/A were significantly inhibited.
CONCLUSION
The ability of BoNT/A to directly inhibit antral muscular contractility suggests a new mechanism for the pharmacologic actions of BoNT-direct inhibition of muscular contraction.
MeSH Terms
Figure
Reference
-
1. Schiavo G, Matteoli M, Montecucco C. Neurotoxins affecting neuroexocytosis. Physiol Rev. 2000. 80:717–766.
Article2. Brin MF. Botulinum toxin: chemistry, pharmacology, toxicity, and immunology. Muscle Nerve Suppl. 1997. 6:S146–S168.
Article3. Bhidayasiri R, Truong DD. Expanding use of botulinum toxin. J Neurol Sci. 2005. 235:1–9.
Article4. Lalli G, Herreros J, Osborne SL, Montecucco C, Rossetto O, Schiavo G. Functional characterisation of tetanus and botulinum neurotoxins binding domains. J Cell Sci. 1999. 112(Pt 16):2715–2724.
Article5. Ji J, Lau H, Sheu L, Diamant NE, Gaisano HY. Distinct regional expression of SNARE proteins in the feline oesophagus. Neurogastroenterol Motil. 2002. 14:383–394.
Article6. Ji J, Salapatek AM, Lau H, Wang G, Gaisano HY, Diamant NE. SNAP-25, a SNARE protein, inhibits two types of K channels in esophageal smooth muscle. Gastroenterology. 2002. 122:994–1006.
Article7. James AN, Ryan JP, Parkman HP. Inhibitory effects of botulinum toxin on pyloric and antral smooth muscle. Am J Physiol Gastrointest Liver Physiol. 2003. 285:G291–G297.8. Karaki H, Urakawa N, Kutsky P. Potassium-induced contraction in smooth muscle. Nihon Heikatsukin Gakkai Zasshi. 1984. 20:427–444.
Article9. Shaari CM, Sanders I. Quantifying how location and dose of botulinum toxin injections affect muscle paralysis. Muscle Nerve. 1993. 16:964–969.
Article10. Lefebvre RA, Baert E, Barbier AJ. Influence of NG-nitro-L-arginine on non-adrenergic non-cholinergic relaxation in the guinea-pig gastric fundus. Br J Pharmacol. 1992. 106:173–179.
Article11. Giladi N. The mechanism of action of botulinum toxin type A in focal dystonia is most probably through its dual effect on efferent (motor) and afferent pathways at the injected site. J Neurol Sci. 1997. 152:132–135.
Article12. Erdal J, Ostergaard L, Fuglsang-Frederiksen A, Werdelin L, Dalager T, Sjö O, et al. Long-term botulinum toxin treatment of cervical dystonia--EMG changes in injected and noninjected muscles. Clin Neurophysiol. 1999. 110:1650–1654.
Article13. Mense S. Neurobiological basis for the use of botulinum toxin in pain therapy. J Neurol. 2004. 251:Suppl 1. I1–I7.
Article14. Dressler D, Saberi F Adib. Botulinum toxin: mechanisms of action. Eur Neurol. 2005. 53:3–9.
Article15. Blasi J, Chapman ER, Link E, Binz T, Yamasaki S, De Camilli P, et al. Botulinum neurotoxin A selectively cleaves the synaptic protein SNAP-25. Nature. 1993. 365:160–163.
Article16. Pasricha PJ, Ravich WJ, Kalloo AN. Botulinum toxin for achalasia. Lancet. 1993. 341:244–245.
Article17. Annese V, Basciani M, Perri F, Lombardi G, Frusciante V, Simone P, et al. Controlled trial of botulinum toxin injection versus placebo and pneumatic dilation in achalasia. Gastroenterology. 1996. 111:1418–1424.
Article18. Truong DD, Jost WH. Botulinum toxin: clinical use. Parkinsonism Relat Disord. 2006. 12:331–355.
Article19. Fernández López F, Conde Freire R, Rios Rios A, García Iglesias J, Caínzos Fernández M, Potel Lesquereux J. Botulinum toxin for the treatment of anal fissure. Dig Surg. 1999. 16:515–518.
Article20. Wiegand H, Erdmann G, Wellhöner HH. 125I-labelled botulinum A neurotoxin: pharmacokinetics in cats after intramuscular injection. Naunyn Schmiedebergs Arch Pharmacol. 1976. 292:161–165.
Article21. Gui D, De Gaetano A, Spada PL, Viggiano A, Cassetta E, Albanese A. Botulinum toxin injected in the gastric wall reduces body weight and food intake in rats. Aliment Pharmacol Ther. 2000. 14:829–834.
Article22. Scott AB, Suzuki D. Systemic toxicity of botulinum toxin by intramuscular injection in the monkey. Mov Disord. 1988. 3:333–335.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Application of Botulinum Toxin in Pain Management
- Exophthalmos Inhibitory Effect of Anti-EPS Serum
- Effects of ryanodine on the intracellular Na+ activity and tension and action potentials of rat and guinea pig cardiac ventricular muscles
- Regulation of L-type Calcium Channel Current by Somatostatin in Guinea-Pig Gastric Myocytes
- Neurotensin enhances gastric motility in antral circular muscle strip of guinea-pig.