Nutr Res Pract.
2019 Aug;13(4):286-294. 10.4162/nrp.2019.13.4.286.
Deficiency or activation of peroxisome proliferator-activated receptor α reduces the tissue concentrations of endogenously synthesized docosahexaenoic acid in C57BL/6J mice
- Affiliations
-
- 1Department of Nutrition, China Medical University, 91 Hsueh-Shih Road, Taichung 404, Taiwan. pmchao@mail.cmu.edu.tw
- 2Graduate Institute of Physiology, National Taiwan University, Taipei 100, Taiwan.
- 3Department of Psychiatry and Mind-Body Interface Laboratory (MBI-Lab), China Medical University Hospital, Taichung 404, Taiwan.
- 4Graduate Institute of Immunology, China Medical University, Taichung 404, Taiwan.
- KMID: 2453106
- DOI: http://doi.org/10.4162/nrp.2019.13.4.286
Abstract
- BACKGROUND/OBJECTIVES
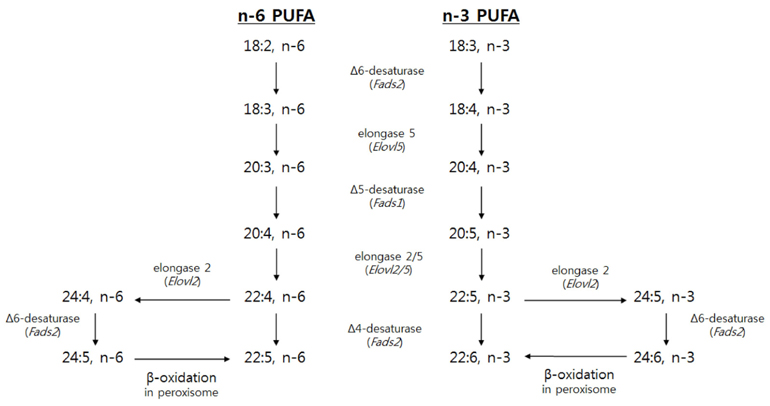
Docosahexaenoic acid (DHA), an n-3 long chain polyunsaturated fatty acid (LCPUFA), is acquired by dietary intake or the in vivo conversion of α-linolenic acid. Many enzymes participating in LCPUFA synthesis are regulated by peroxisome proliferator-activated receptor alpha (PPARα). Therefore, it was hypothesized that the tissue accretion of endogenously synthesized DHA could be modified by PPARα.
MATERIALS/METHODS
The tissue DHA concentrations and mRNA levels of genes participating in DHA biosynthesis were compared among PPARα homozygous (KO), heterozygous (HZ), and wild type (WT) mice (Exp I), and between WT mice treated with clofibrate (PPARα agonist) or those not treated (Exp II). In ExpII, the expression levels of the proteins associated with DHA function in the brain cortex and retina were also measured. An n3-PUFA depleted/replenished regimen was applied to mitigate the confounding effects of maternal DHA.
RESULTS
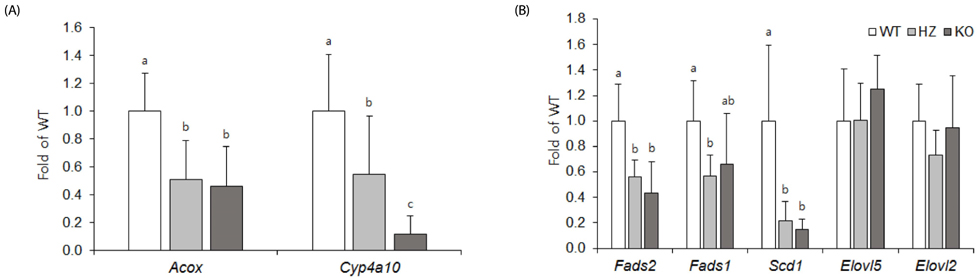
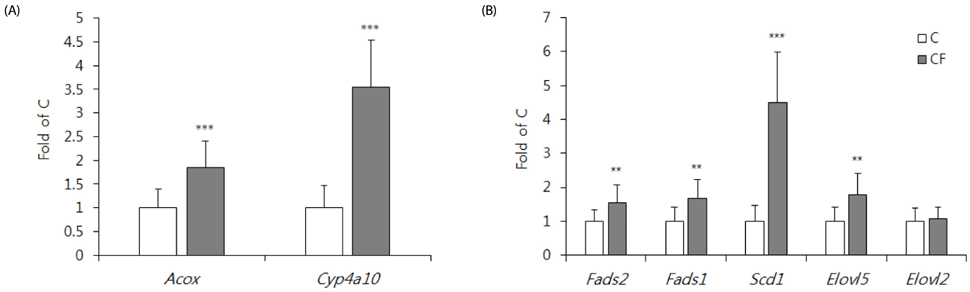
PPARα ablation reduced the hepatic Acox, Fads1, and Fads2 mRNA levels, as well as the DHA concentration in the liver, but not in the brain cortex. In contrast, PPARα activation increased hepatic Acox, Fads1, Fads2 and Elovl5 mRNA levels, but reduced the DHA concentrations in the liver, retina, and phospholipid of brain cortex, and decreased mRNA and protein levels of the brain-derived neurotrophic factor in brain cortex.
CONCLUSIONS
LCPUFA enzyme expression was altered by PPARα. Either PPARα deficiency or activation-decreased tissue DHA concentration is a stimulus for further studies to determine the functional significance.
Keyword
MeSH Terms
Figure
Reference
-
1. Molloy C, Doyle LW, Makrides M, Anderson PJ. Docosahexaenoic acid and visual functioning in preterm infants: a review. Neuropsychol Rev. 2012; 22:425–437.
Article2. Echeverría F, Valenzuela R, Catalina Hernandez-Rodas M, Valenzuela A. Docosahexaenoic acid (DHA), a fundamental fatty acid for the brain: new dietary sources. Prostaglandins Leukot Essent Fatty Acids. 2017; 124:1–10.
Article3. Salem N Jr, Litman B, Kim HY, Gawrisch K. Mechanisms of action of docosahexaenoic acid in the nervous system. Lipids. 2001; 36:945–959.
Article4. Iizuka-Hishikawa Y, Hishikawa D, Sasaki J, Takubo K, Goto M, Nagata K, Nakanishi H, Shindou H, Okamura T, Ito C, Toshimori K, Sasaki T, Shimizu T. Lysophosphatidic acid acyltransferase 3 tunes the membrane status of germ cells by incorporating docosahexaenoic acid during spermatogenesis. J Biol Chem. 2017; 292:12065–12076.
Article5. Harris WS, Bulchandani D. Why do omega-3 fatty acids lower serum triglycerides? Curr Opin Lipidol. 2006; 17:387–393.
Article6. Geleijnse JM, Giltay EJ, Grobbee DE, Donders AR, Kok FJ. Blood pressure response to fish oil supplementation: metaregression analysis of randomized trials. J Hypertens. 2002; 20:1493–1499.
Article7. Horrocks LA, Yeo YK. Health benefits of docosahexaenoic acid (DHA). Pharmacol Res. 1999; 40:211–225.
Article8. Lin PY, Chiu CC, Huang SY, Su KP. A meta-analytic review of polyunsaturated fatty acid compositions in dementia. J Clin Psychiatry. 2012; 73:1245–1254.
Article9. McNamara RK, Hahn CG, Jandacek R, Rider T, Tso P, Stanford KE, Richtand NM. Selective deficits in the omega-3 fatty acid docosahexaenoic acid in the postmortem orbitofrontal cortex of patients with major depressive disorder. Biol Psychiatry. 2007; 62:17–24.
Article10. McNamara RK, Jandacek R, Rider T, Tso P, Stanford KE, Hahn CG, Richtand NM. Deficits in docosahexaenoic acid and associated elevations in the metabolism of arachidonic acid and saturated fatty acids in the postmortem orbitofrontal cortex of patients with bipolar disorder. Psychiatry Res. 2008; 160:285–299.
Article11. Chang JP, Su KP, Mondelli V, Pariante CM. Omega-3 polyunsaturated fatty acids in youths with attention deficit hyperactivity disorder: a systematic review and meta-analysis of clinical trials and biological studies. Neuropsychopharmacology. 2018; 43:534–545.
Article12. Park HG, Park WJ, Kothapalli KS, Brenna JT. The fatty acid desaturase 2 (FADS2) gene product catalyzes Δ4 desaturation to yield n-3 docosahexaenoic acid and n-6 docosapentaenoic acid in human cells. FASEB J. 2015; 29:3911–3919.
Article13. Pauter AM, Olsson P, Asadi A, Herslöf B, Csikasz RI, Zadravec D, Jacobsson A. Elovl2 ablation demonstrates that systemic DHA is endogenously produced and is essential for lipid homeostasis in mice. J Lipid Res. 2014; 55:718–728.
Article14. Voss A, Reinhart M, Sankarappa S, Sprecher H. The metabolism of 7,10,13,16,19-docosapentaenoic acid to 4,7,10,13,16,19-docosahexaenoic acid in rat liver is independent of a 4-desaturase. J Biol Chem. 1991; 266:19995–20000.
Article15. Moore SA, Hurt E, Yoder E, Sprecher H, Spector AA. Docosahexaenoic acid synthesis in human skin fibroblasts involves peroxisomal retroconversion of tetracosahexaenoic acid. J Lipid Res. 1995; 36:2433–2443.
Article16. Ferdinandusse S, Denis S, Mooijer PA, Zhang Z, Reddy JK, Spector AA, Wanders RJ. Identification of the peroxisomal β-oxidation enzymes involved in the biosynthesis of docosahexaenoic acid. J Lipid Res. 2001; 42:1987–1995.
Article17. Wang Y, Botolin D, Christian B, Busik J, Xu J, Jump DB. Tissue-specific, nutritional, and developmental regulation of rat fatty acid elongases. J Lipid Res. 2005; 46:706–715.
Article18. Tang C, Cho HP, Nakamura MT, Clarke SD. Regulation of human Δ-6 desaturase gene transcription: identification of a functional direct repeat-1 element. J Lipid Res. 2003; 44:686–695.
Article19. Matsuzaka T, Shimano H, Yahagi N, Amemiya-Kudo M, Yoshikawa T, Hasty AH, Tamura Y, Osuga J, Okazaki H, Iizuka Y, Takahashi A, Sone H, Gotoda T, Ishibashi S, Yamada N. Dual regulation of mouse Delta(5)- and Delta(6)-desaturase gene expression by SREBP-1 and PPARalpha. J Lipid Res. 2002; 43:107–114.
Article20. Qi C, Zhu Y, Reddy JK. Peroxisome proliferator-activated receptors, coactivators, and downstream targets. Cell Biochem Biophys. 2000; 32:187–204.
Article21. Oosterveer MH, Grefhorst A, van Dijk TH, Havinga R, Staels B, Kuipers F, Groen AK, Reijngoud DJ. Fenofibrate simultaneously induces hepatic fatty acid oxidation, synthesis, and elongation in mice. J Biol Chem. 2009; 284:34036–34044.
Article22. Tian Q, Grzemski FA, Panagiotopoulos S, Ahokas JT. Peroxisome proliferator-activated receptor alpha agonist, clofibrate, has profound influence on myocardial fatty acid composition. Chem Biol Interact. 2006; 160:241–251.
Article23. Madsen L, Frøyland L, Dyrøy E, Helland K, Berge RK. Docosahexaenoic and eicosapentaenoic acids are differently metabolized in rat liver during mitochondria and peroxisome proliferation. J Lipid Res. 1998; 39:583–593.
Article24. Reeves PG, Nielsen FH, Fahey GC Jr. AIN-93 purified diets for laboratory rodents: final report of the American Institute of Nutrition ad hoc writing committee on the reformulation of the AIN-76A rodent diet. J Nutr. 1993; 123:1939–1951.
Article25. Blank C, Neumann MA, Makrides M, Gibson RA. Optimizing DHA levels in piglets by lowering the linoleic acid to alpha-linolenic acid ratio. J Lipid Res. 2002; 43:1537–1543.
Article26. Chang YY, Su HM, Chen SH, Hsieh WT, Chyuan JH, Chao PM. Roles of peroxisome proliferator-activated receptor α in bitter melon seed oil-corrected lipid disorders and conversion of α-eleostearic acid into rumenic acid in C57BL/6J mice. Nutrients. 2016; 8:E805.
Article27. Nguyen LN, Ma D, Shui G, Wong P, Cazenave-Gassiot A, Zhang X, Wenk MR, Goh EL, Silver DL. Mfsd2a is a transporter for the essential omega-3 fatty acid docosahexaenoic acid. Nature. 2014; 509:503–506.
Article28. Wong BH, Chan JP, Cazenave-Gassiot A, Poh RW, Foo JC, Galam DL, Ghosh S, Nguyen LN, Barathi VA, Yeo SW, Luu CD, Wenk MR, Silver DL. Mfsd2a is a transporter for the essential ω-3 fatty acid docosahexaenoic acid (DHA) in eye and is important for photoreceptor cell development. J Biol Chem. 2016; 291:10501–10514.
Article29. Balogun KA, Cheema SK. The expression of neurotrophins is differentially regulated by ω-3 polyunsaturated fatty acids at weaning and postweaning in C57BL/6 mice cerebral cortex. Neurochem Int. 2014; 66:33–42.
Article30. Squinto SP, Stitt TN, Aldrich TH, Davis S, Blanco SM, Radziejewski C, Glass DJ, Masiakowski P, Furth ME, Valenzuela DM, Distefano PS, Yancopoulos GD. trkB encodes a functional receptor for brain-derived neurotrophic factor and neurotrophin-3 but not nerve growth factor. Cell. 1991; 65:885–893.
Article31. Garelli A, Rotstein NP, Politi LE. Docosahexaenoic acid promotes photoreceptor differentiation without altering Crx expression. Invest Ophthalmol Vis Sci. 2006; 47:3017–3027.
Article32. Williams CM, Burdge G. Long-chain n-3 PUFA: plant v. marine sources. Proc Nutr Soc. 2006; 65:42–50.
Article33. Miller CW, Ntambi JM. Peroxisome proliferators induce mouse liver stearoyl-CoA desaturase 1 gene expression. Proc Natl Acad Sci U S A. 1996; 93:9443–9448.
Article34. Kitson AP, Stroud CK, Stark KD. Elevated production of docosahexaenoic acid in females: potential molecular mechanisms. Lipids. 2010; 45:209–224.
Article35. Pawlosky R, Hibbeln J, Lin Y, Salem N. n-3 fatty acid metabolism in women. Br J Nutr. 2003; 90:993–994.
Article36. Querques G, Forte R, Souied EH. Retina and omega-3. J Nutr Metab. 2011; 2011:748361.
Article37. Hill RE, Favor J, Hogan BL, Ton CC, Saunders GF, Hanson IM, Prosser J, Jordan T, Hastie ND, van Heyningen V. Mouse small eye results from mutations in a paired-like homeobox-containing gene. Nature. 1991; 354:522–525.
Article38. Furukawa T, Morrow EM, Li T, Davis FC, Cepko CL. Retinopathy and attenuated circadian entrainment in Crx-deficient mice. Nat Genet. 1999; 23:466–470.
Article39. SanGiovanni JP, Chew EY. The role of omega-3 long-chain polyunsaturated fatty acids in health and disease of the retina. Prog Retin Eye Res. 2005; 24:87–138.
Article40. Grossfield A, Feller SE, Pitman MC. A role for direct interactions in the modulation of rhodopsin by omega-3 polyunsaturated lipids. Proc Natl Acad Sci U S A. 2006; 103:4888–4893.
Article41. Miller DS, Nobmann SN, Gutmann H, Toeroek M, Drewe J, Fricker G. Xenobiotic transport across isolated brain microvessels studied by confocal microscopy. Mol Pharmacol. 2000; 58:1357–1367.
Article42. Maes M, Delanghe J, Meltzer HY, Scharpé S, D'Hondt P, Cosyns P. Lower degree of esterification of serum cholesterol in depression: relevance for depression and suicide research. Acta Psychiatr Scand. 1994; 90:252–258.
Article43. Brunner J, Parhofer KG, Schwandt P, Bronisch T. Cholesterol, essential fatty acids, and suicide. Pharmacopsychiatry. 2002; 35:1–5.
Article44. Kunugi H, Takei N, Aoki H, Nanko S. Low serum cholesterol in suicide attempters. Biol Psychiatry. 1997; 41:196–200.
Article45. Papassotiropoulos A, Hawellek B, Frahnert C, Rao GS, Rao ML. The risk of acute suicidality in psychiatric inpatients increases with low plasma cholesterol. Pharmacopsychiatry. 1999; 32:1–4.
Article46. Muldoon MF, Manuck SB, Matthews KA. Lowering cholesterol concentrations and mortality: a quantitative review of primary prevention trials. BMJ. 1990; 301:309–314.
Article47. Su KP, Tsai SY, Huang SY. Cholesterol, depression and suicide. Br J Psychiatry. 2000; 176:399.
Article48. Roy A, Jana M, Corbett GT, Ramaswamy S, Kordower JH, Gonzalez FJ, Pahan K. Regulation of cyclic AMP response element binding and hippocampal plasticity-related genes by peroxisome proliferator-activated receptor α. Cell Reports. 2013; 4:724–737.
Article49. Xu H, You Z, Wu Z, Zhou L, Shen J, Gu Z. WY14643 attenuates the scopolamine-induced memory impairments in mice. Neurochem Res. 2016; 41:2868–2879.
Article50. Jiang B, Wang YJ, Wang H, Song L, Huang C, Zhu Q, Wu F, Zhang W. Antidepressant-like effects of fenofibrate in mice via the hippocampal brain-derived neurotrophic factor signalling pathway. Br J Pharmacol. 2017; 174:177–194.
Article51. Ren H, Aleksunes LM, Wood C, Vallanat B, George MH, Klaassen CD, Corton JC. Characterization of peroxisome proliferator-activated receptor alpha--independent effects of PPARalpha activators in the rodent liver: di-(2-ethylhexyl) phthalate also activates the constitutive-activated receptor. Toxicol Sci. 2010; 113:45–59.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Peroxisome Proliferator Activated Receptor-delta (PPAR-delta)
- Peroxisome Proliferator-activated Receptors (PPARs) in Diabetic Nephropathy
- Refocusing Peroxisome Proliferator Activated Receptor-alpha: A New Insight for Therapeutic Roles in Diabetes
- Phenotype of peroxisome proliferator-activated receptor-alpha(PPARalpha)deficient mice on mixed background fed high fat diet
- Peroxisome Proliferator-Activated Receptor (PPAR) alpha/gamma Agonist