J Neurocrit Care.
2019 Jun;12(1):55-63. 10.18700/jnc.190083.
Primary central nervous system lymphoma with intramedullary spinal cord involvement mimicking inflammatory demyelinating disease
- Affiliations
-
- 1Department of Neurology, Chonnam National University Hospital, Chonnam National University Medical School, Gwangju, Republic of Korea. nts0022@hanmail.net
- 2Department of Neurology, Massachusetts General Hospital, Harvard Medical School, Boston, MA, USA. mlevy11@mgh.harvard.edu
- 3Department of Pathology, Chonnam National University Medical School, Gwangju, Republic of Korea.
- 4Department of Nuclear Medicine, Chonnam National University Hospital, Chonnam National University Medical School, Gwangju, Republic of Korea.
- 5Department of Radiology, Chonnam National University Hospital, Chonnam National University Medical School, Gwangju, Republic of Korea.
- KMID: 2452828
- DOI: http://doi.org/10.18700/jnc.190083
Abstract
- BACKGROUND
Spinal cord involvement of primary central nervous system lymphoma (PCNSL) is rare in a young immunocompetent patient and can be misdiagnosed as an inflammatory demyelinating disease (IDD) of the central nervous system.
CASE REPORT
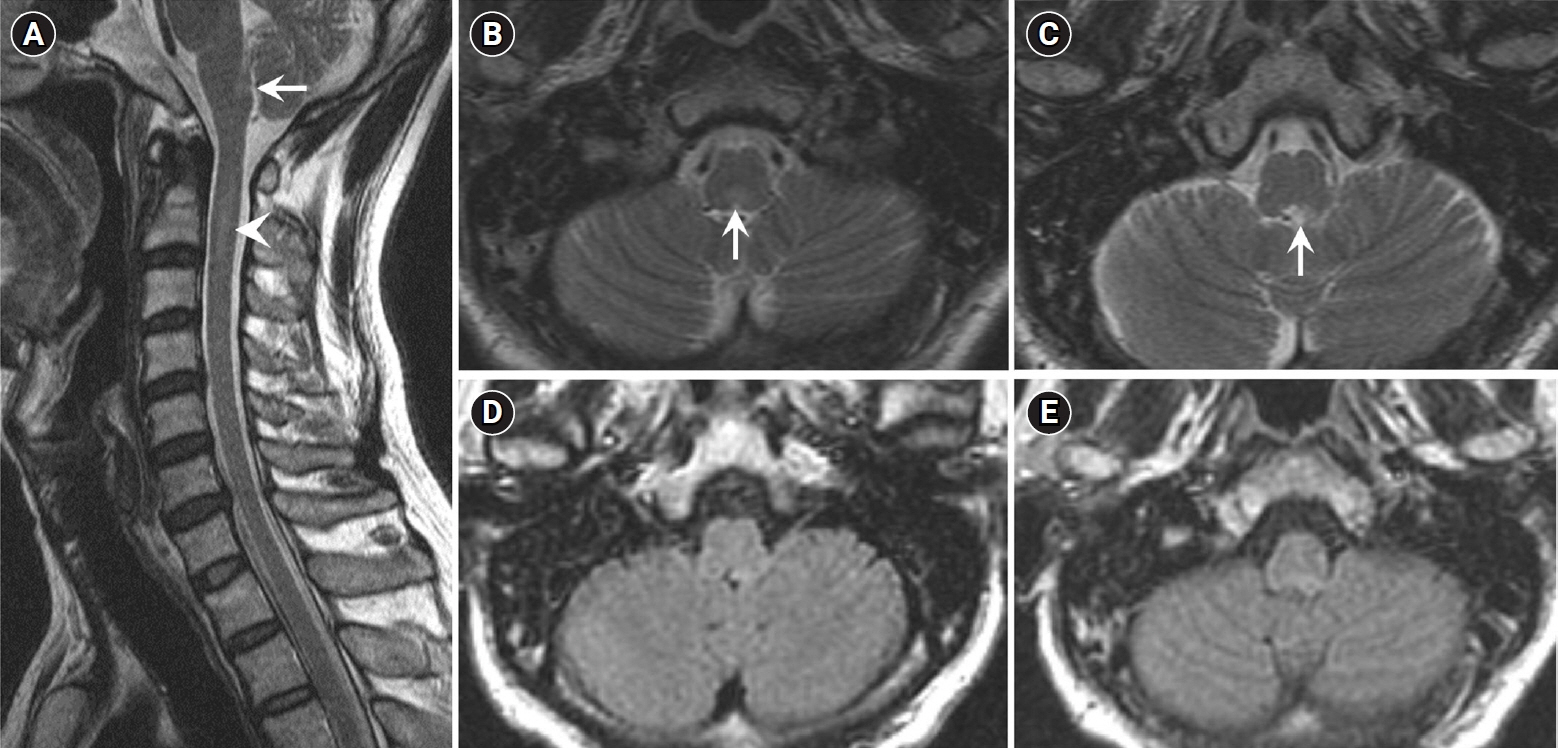
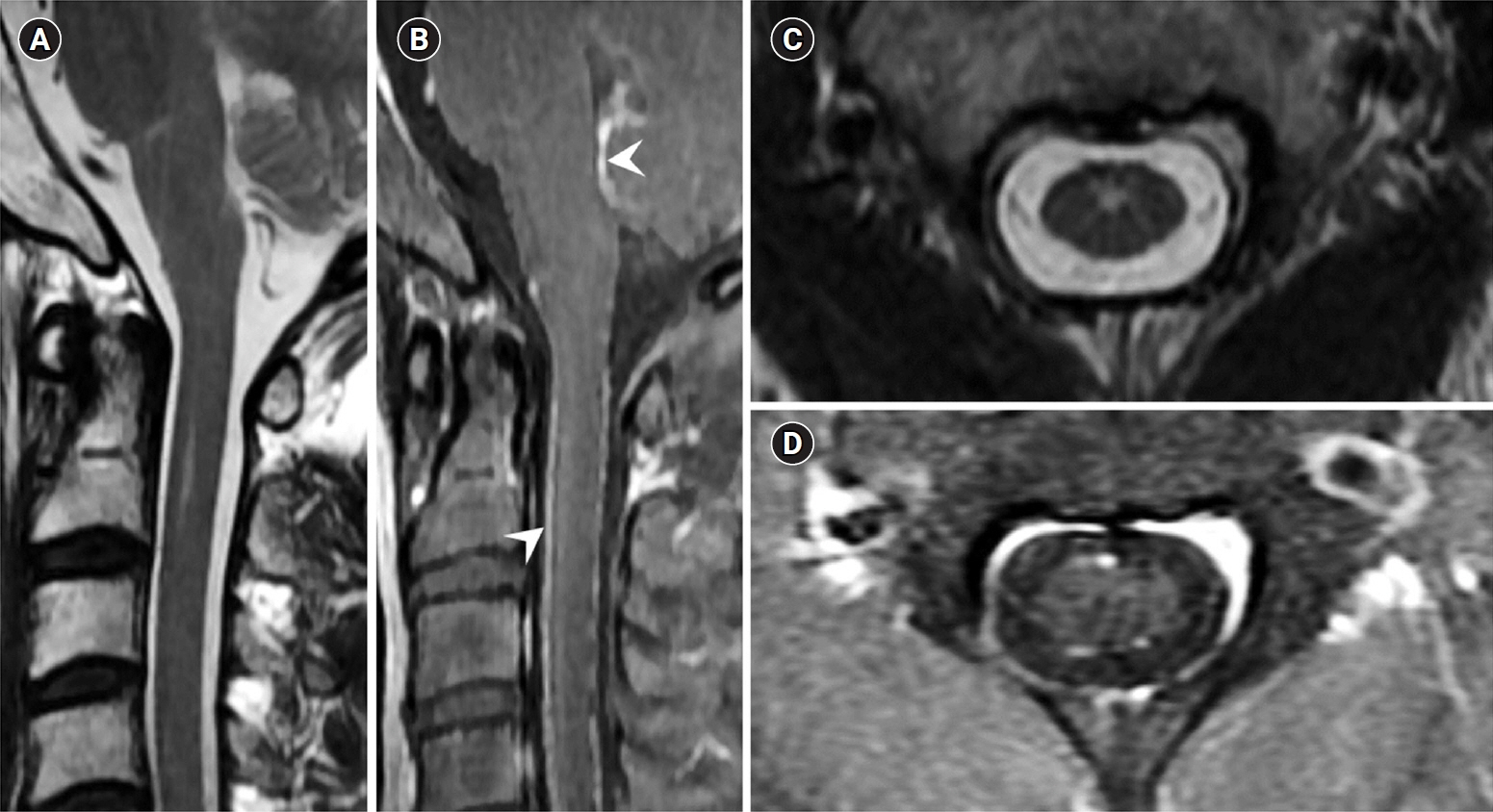
We report a case of PCNSL mimicking IDD in a previously healthy 46-year-old man with weakness in both hands for 1 week. Magnetic resonance imaging (MRI) of the cervical spinal cord revealed contrast-enhancing intraparenchymal and leptomeningeal lesions in the cervical spinal cord and medulla oblongata. Cerebrospinal fluid analysis revealed pleocytosis (37/mm³). The patient's symptoms and lesions improved with corticosteroid treatment. However, he developed semicomatose mentality 5 months later. Brain MRI, ventricular biopsy, and ¹â¸F -flurodeoxyglucose positron emission tomography/computed tomography confirmed PCNSL. The patient deceased 3 months later, despite high-dose methotrexate chemotherapy.
CONCLUSION
Persistent gadolinium-enhancing MRI lesions along the ventricular regions and spinal leptomeninges could differentiate PCNSL involving the spinal cord from IDD in the early stages of the disease.
Keyword
MeSH Terms
Figure
Reference
-
1. Grommes C, DeAngelis LM. Primary CNS lymphoma. J Clin Oncol. 2017; 35:2410–8.
Article2. Hochberg FH, Baehring JM, Hochberg EP. Primary CNS lymphoma. Nat Clin Pract Neurol. 2007; 3:24–35.
Article3. Sierra del Rio M, Rousseau A, Soussain C, Ricard D, Hoang-Xuan K. Primary CNS lymphoma in immunocompetent patients. Oncologist. 2009; 14:526–39.
Article4. Herrlinger U, Weller M, Küker W. Primary CNS lymphoma in the spinal cord: clinical manifestations may precede MRI detectability. Neuroradiology. 2002; 44:239–44.5. Flanagan EP, O'Neill BP, Porter AB, Lanzino G, Haberman TM, Keegan BM. Primary intramedullary spinal cord lymphoma. Neurology. 2011; 77:784–91.
Article6. Elavarasi A, Dash D, Warrier AR, Bhatia R, Kumar L, Jain D, et al. Spinal cord involvement in primary CNS lymphoma. J Clin Neurosci. 2018; 47:145–8.
Article7. DeAngelis LM. Primary central nervous system lymphoma imitates multiple sclerosis. J Neurooncol. 1990; 9:177–81.
Article8. Kim SM, Kim SJ, Lee HJ, Kuroda H, Palace J, Fujihara K. Differential diagnosis of neuromyelitis optica spectrum disorders. Ther Adv Neurol Disord. 2017; 10:265–89.
Article9. Scott BJ, Douglas VC, Tihan T, Rubenstein JL, Josephson SA. A systematic approach to the diagnosis of suspected central nervous system lymphoma. JAMA Neurol. 2013; 70:311–9.
Article10. Han CH, Batchelor TT. Primary central nervous system lymphoma. Continuum (Minneap Minn). 2017; 23(6, Neuro-oncology):1601–18.
Article11. Mohile NA, Deangelis LM, Abrey LE. The utility of body FDG PET in staging primary central nervous system lymphoma. Neuro Oncol. 2008; 10:223–8.
Article12. Chiavazza C, Pellerino A, Ferrio F, Cistaro A, Soffietti R, Rudà R. Primary CNS lymphomas: challenges in diagnosis and monitoring. Biomed Res Int. 2018; 2018:3606970.
Article13. Bromberg JE, Breems DA, Kraan J, Bikker G, van der Holt B, Smitt PS, et al. CSF flow cytometry greatly improves diagnostic accuracy in CNS hematologic malignancies. Neurology. 2007; 68:1674–9.
Article14. Cordone I, Masi S, Carosi M, Vidiri A, Marchesi F, Marino M, et al. Brain stereotactic biopsy flow cytometry for central nervous system lymphoma characterization: advantages and pitfalls. J Exp Clin Cancer Res. 2016; 35:128.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Disseminated Tuberculosis of Central Nervous System : Spinal Intramedullary and Intracranial Tuberculomas
- Primary central nervous system lymphoma in the brainstem and cervical spinal cord: a case report and literature review
- A Case of Spinal Cord Glioblastoma Multiforme with Intracranial Metastasis
- Spinal Cord Neurosarcoidosis after Cervical Compressive Myelopathy
- Primary Peripheral Gamma Delta T-Cell Lymphoma of the Central Nervous System: Report of a Case Involving the Intramedullary Spinal Cord and Presenting with Myelopathy