Nat Prod Sci.
2019 Jun;25(2):157-164. 10.20307/nps.2019.25.2.157.
Evaluation of Acute and Sub-acute Oral Toxicity Effect of Aquilaria malaccensis Leaves Aqueous Extract in Male ICR Mice
- Affiliations
-
- 1Department of Biology, Faculty of Science and Mathematics, Universiti Pendidikan Sultan Idris, 35900 Tanjong Malim, Perak, Malaysia. haniza@fsmt.upsi.edu.my
- KMID: 2452786
- DOI: http://doi.org/10.20307/nps.2019.25.2.157
Abstract
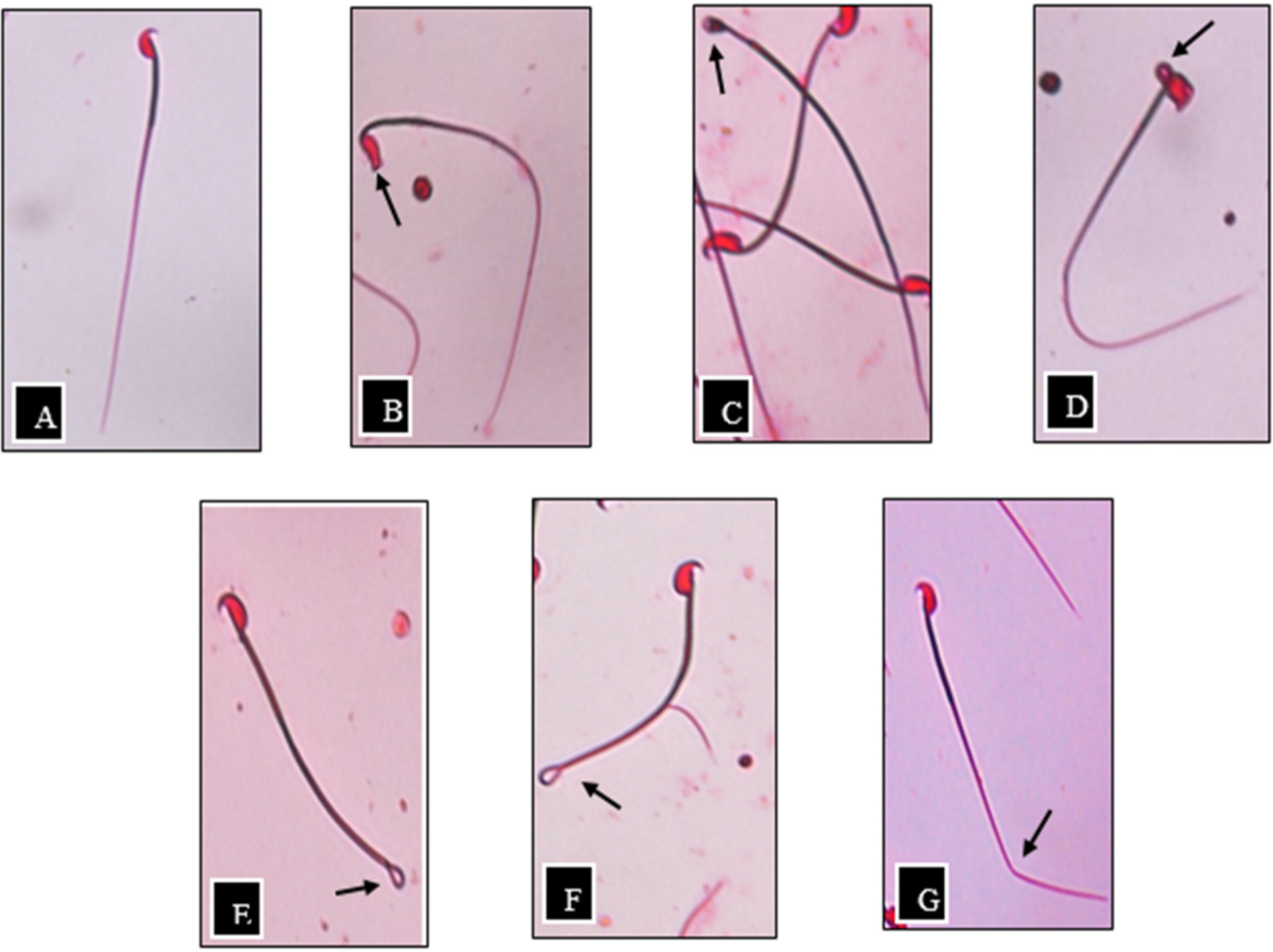
- The study was conducted to investigate the acute and sub-acute toxicity effect of Aquilaria malaccensis leaves aqueous extract (AEAM) towards male ICR mice in terms of body weight, relative organ weight, mortality rate and sperm parameters. In acute toxicity study, a single dose at of 2000 mg/kg was performed. In sub-acute toxicity study, the mice were received normal saline (control group), 50, 100, 150, 200, 500, or 1000 mg/kg of AEAM orally for 21 days of treatment. In sub-acute toxicity study, the number of abnormal sperm were significantly decreased in AEAM 100, 150, 200, 500, and 1000 when compared to the control group. While, the motility of sperm were found to be significantly increased in AEAM 100, 150, 200, and 1000 as compared to the control group. No mortality was recorded in the control group and treated groups in both toxicity studies except for one mouse from AEAM 1000 group. However, the mild sedative effect in terms of the tendency to sleep was clearly noticeable in both toxicity studies. Results indicated that the AEAM can be one of the useful alternative medicine to enhance fertility rate by increasing healthy sperm production.
MeSH Terms
Figure
Reference
-
(1). Redzuan Nul Hakim A. R., Muhammad Lokman M. I., Afzan M. Y., Azantee Yazmie A. W., Hussin M., Roszaman R. J.Biotechnol. Strategic Health Res. 2017; 1:17–23.(2). Parandin R., Yousofvand N., Ghorbani R.Iran J. Reprod. Med. 2012; 10:355–362.(3). Agnes V. F., Akbarsha M. A.Food Chem. Toxicol. 2003; 41:119–130.(4). Chauhan N. S., Dixit V. K.Int. J. Appl. Res. Nat. Prod. 2008; 1:26–31.(5). Ekaluo U. B., Erem F. A., Omeje I. S., Ikpeme E. V., Ibiang Y. B., Ekanem B. E.IOSR J. Environ. Sci. Toxicol. Food Technol. 2013; 3:21–23.(6). Wahab N. A., Mokhtar N. M., Halim W. N., Das S.Clinics. 2010; 65:93–98.(7). Mahmoud A. S. F., Noor M. M.AIP Conf. Proc. 2013; 1571:227–233.(8). Nor-Raidah R., Mahanem M. N.Malays. Appl. Biol. 2015; 44:125–131.(9). Hakim P., Sani H. A., Noor M. M.Malaysian J. Biochem. Mol. Biol. 2008; 16:10–14.(10). Giribabu N., Kumar K. E., Rekha S. S., Muniandy S., Salleh N.BMC Complement. Altern. Med. 2014; 14:291.(11). Parhizkar S., Yusoff M. J., Dollah M. A.Adv. Pharm. Bull. 2013; 3:345–352.(12). Idris M. H., Budin S. B., Osman M., Mohamed J.EXCLI J. 2012; 11:659–669.(13). Fatmawati , Hidayat R.Eur. J. Pharm. Med. Res. 2016; 3:46–49.(14). Bahrani H., Mohamad J., Paydar M. J., Rothan H. A.Curr. Alzheimer Res. 2014; 11:206–214.(15). Hara H., Ise Y., Morimoto N., Shimazawa M., Ichihashi K., Ohyama M., Iinuma M.Biosci. Biotechnol. Biochem. 2008; 72:335–345.(16). Kakino M., Tazawa S., Maruyama H., Tsuruma K., Araki Y., Shimazawa M., Hara H.BMC Complement. Altern. Med. 2010; 10:68.(17). Zhou M., Wang H., Suolangjiba Kou J., Yu B. J.Ethnopharmacol. 2008; 117:345–350.(18). Dash M., Patra J. K., Panda P. P. Afr. J.Biotechnol. 2008; 7:3531–3534.(19). Begum Y.Pharma Tutor. 2016; 4:47–50.(20). Pranakhon R., Pannangpetch P., Aromdee C. Songklanakarin. J.Sci. Technol. 2011; 33:405–410.(21). Ukwuani A. N., Abubakar M. G., Hassan S. W., Agaie B. M.Int. J. Pharm. Sci. Drug Res. 2012; 4:245–249.(22). Ali Khairullah Z., Hazilawati H., Hutheyfa S., Mohd Rosly S., Sithambaram S., Hemn Hasan O.Asian J. Pharm. Clin. Res. 2015; 8:400–408.(23). Anisuzzaman A. S. M., Sugimoto N., Sadik G., Gafur M. A. Pak. J.Biol. Sci. 2001; 4:1012–1015.(24). Kazmi I., Afzal M., Rahman M., Gupta G., Anwar F. Asian Pac. J.Trop. Dis. 2012; 2:S841–S845.(25). Carro-Juárez M., Franco M. Á., Rodriguez-Peña Mde. L. J.Evid. Based Complement. Altern. Med. 2014; 19:43–50.(26). Wil N. N. A. N., Omar N. M., Ibrahim N. A., Tajuddin S. N. J.Chem. Pharm. Res. 2014; 6:688–693.(27). Ghan S. Y., Chin J. H., Thoo Y. Y., Yim H. S., Ho C. W.Int. J. Pharm. Sci. Res. 2016; 7:1456–1461.
Article(28). Veeresh Kumar P., Gupta V. R. M. J.Phytopharmacol. 2017; 6:178–182.(29). OECD/OCDE, OECD Guidelines for the testing of chemicals 407. 2008.(30). Erhirhie E. O., Ekene N. E., Ajaghaku D. L. J.Nat. Sci. Res. 2014; 4:100–106.(31). Ali R., Ali R., Jaimini A., Nishad D. K., Mittal G., Chaurasia O. P., Kumar R., Bhatnagar A., Singh S. B.Indian J. Pharmacol. 2012; 44:504–508.(32). Arsad S. S., Esa N. M., Hamzah H., Othman F. J.Med. Plant Res. 2013; 7:3030–3040.(33). Aniagu S. O., Nwinyi F. C., Akumka D. D., Ajoku G. A., Dzarma S., Izebe K. S., Ditse M., Nwaneri P. E. C., Wambebe C., Gamaniel K. Afr. J.Biotechnol. 2005; 4:72–78.(34). Ilgın S., Aydoğan-Kılıç G., Baysal M., Kılıç V, Ardıç M., Uçarcan Ş., Atlı Ö.Oxid. Med. Cell. Longev. 2018; 2018:7196142.(35). Sönmez M., Türk G., Yüce A.Theriogenology. 2005; 63:2063–2072.(36). El-Kashoury A. A., Salama A. F., Selim A. I., Mohamed R. A.Life Sci. J. 2010; 7:5–19.(37). Akunna G. G., Saalu L. C., Ogunlade B., Ojewale A. O., Enye L. A. Am. J.Res. Commun. 2013; 1:123–142.(38). Sakr S. A., Zowail M. E., Marzouk A. M.Anat. Cell. Biol. 2014; 47:171–179.(39). Takeda N., Yoshinaga K., Furushima K., Takamune K., Li Z., Abe S. I., Aizawa S., Yamamura K.Sci. Rep. 2016; 6:27409.(40). Lucio R. A., Tlachi-López J. L., Eguibar J. R., Ågmo A.Physiol. Behav. 2013; 110:73–79.(41). Neergheen-Bhujun V. S.Biomed. Res. Int. 2013; 2013:804086.(42). Pingale S. S., Ganpat M. A., Gawali S. Int. Res. J.Pharm. 2011; 2:263–266.(43). Ellacott K. L., Morton G. J., Woods S. C., Tso P., Schwartz M. W.Cell. Metab. 2010; 12:10–17.(44). Sattayasai J., Bantadkit J., Aromdee C., Lattmann E., Airarat W. J.Ayurveda Integr. Med. 2012; 3:175–179.(45). Sanyal S., Maity P., Pradhan A., Bepari M., Dey S. K., Roy T., Choudhury S. T.Toxicol. Forensic Med. 2016; 1:54–64.(46). Sharif H. B., Mukhtar M. D., Mustapha Y., Baba G., Lawal A. O.Adv. Pharm. 2015; 2015:1–9.(47). Shahraki M. R., Shahraki S., Arab M. R., Shahrakipour M.Zahedan J. Res. Med. Sci. 2015; 17:42–46.(48). Chen D., Bi D., Song Y. L., Tu P. F. Chin. J.Nat. Med. 2012; 10:287–291.(49). Yang L., Qiao L., Xie D., Yuan Y., Chen N., Dai J., Guo S.Phytochemistry. 2012; 76:92–97.(50). Peng K., Mei W. L., Zhao Y. X., Tan L. H., Wang Q. H., Dai H. F. J.Asian Nat. Prod. Res. 2011; 13:951–955.(51). Chen H. Q., Wei J. H., Yang J. S., Zhang Z., Yang Y., Gao Z. H., Sui C., Gong B.Chem. Biodivers. 2012; 9:236–250.(52). Khalil A. S., Rahim A. A., Taha K. K., Abdallah K. B.J Appl. Indus. Sci. 2013; 1:78–88.(53). Sheweita S. A., Tilmisany A. M., Al-Sawaf H.Curr. Drug Metab. 2005; 6:495–501.(54). Thakur M., Chauhan N. S., Bhargava S., Dixit V. K.Arch. Sex. Behav. 2009; 38:1009–1015.(55). Cheah Y., Yang W.Adv. Biosci. Biotechnol. 2011; 2:182–197.(56). Adenubi O. T., Raji Y., Awe E. O., Makinde J. M.Sci. World J. 2010; 5:1–6.(57). Yousef M. I., Salama A. F.Food Chem. Toxicol. 2009; 47:1168–1175.(59). Agarwal A., Sekhon L. H.Hum. Fertil. 2010; 13:217–225.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Acute and 13-week subchronic toxicological evaluations of turanose in mice
- Mouse Single Oral Dose Toxicity Test of Bupleuri Radix Aqueous Extracts
- An Experimental Study on the Protective Effects of Ginseng Extract to Oxygen Toxicity
- Acute toxicity of benzalkonium chloride in Balb/c mice following intratracheal instillation and oral administration
- Development of chitosan-catechol conjugates as mucoadhesive polymer: assessment of acute oral toxicity in mice