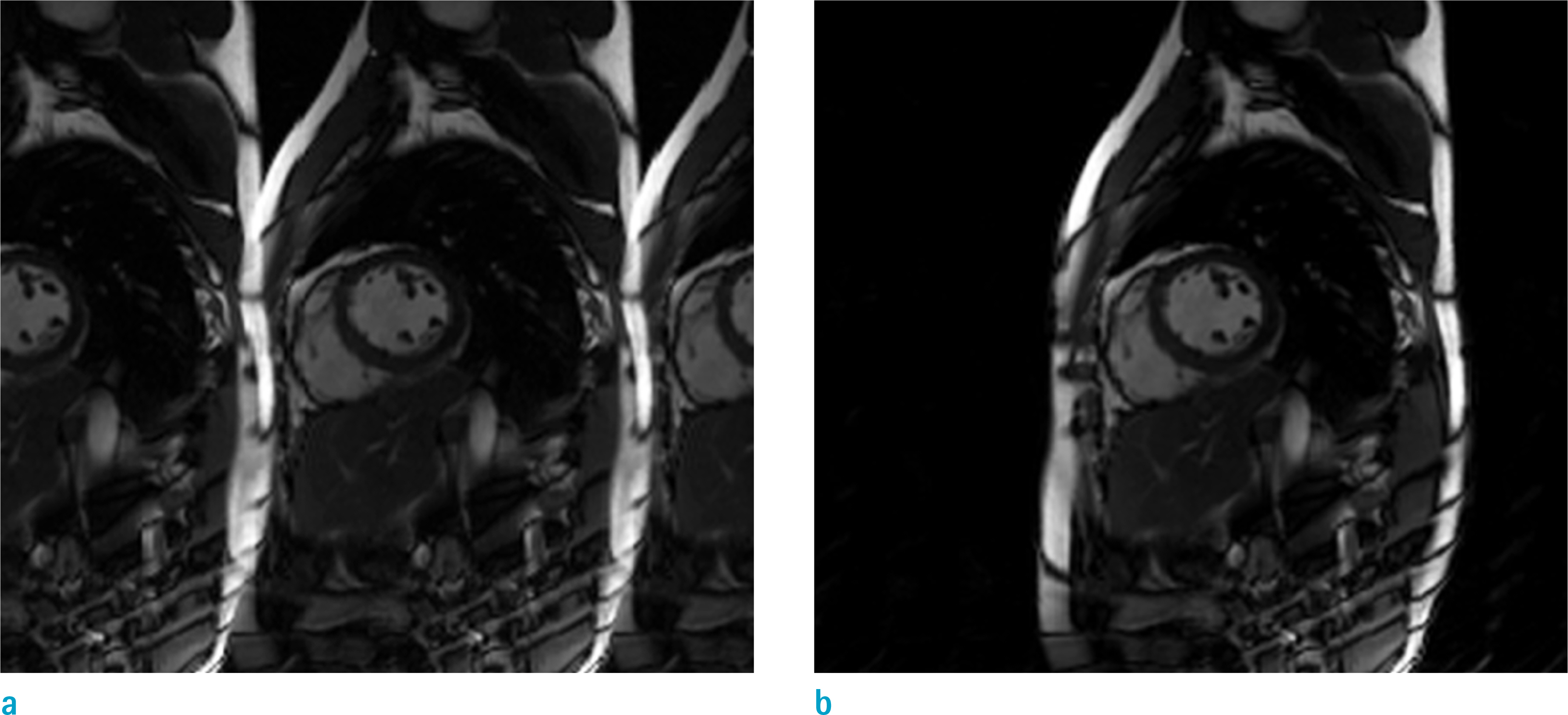
Investig Magn Reson Imaging.
2019 Jun;23(2):114-124. 10.13104/imri.2019.23.2.114.
Biases in the Assessment of Left Ventricular Function by Compressed Sensing Cardiovascular Cine MRI
- Affiliations
-
- 1Department of Electrical Engineering, Kwangwoon University, Seoul, Korea. cbahn@kw.ac.kr
- 2Department of Radiology, Severance Hospital, Yonsei University, Seoul, Korea.
- 3Developing Brain Research Laboratory, Children's National Health System, Washington, DC, USA.
- KMID: 2452525
- DOI: http://doi.org/10.13104/imri.2019.23.2.114
Abstract
- PURPOSE
We investigate biases in the assessments of left ventricular function (LVF), by compressed sensing (CS)-cine magnetic resonance imaging (MRI).
MATERIALS AND METHODS
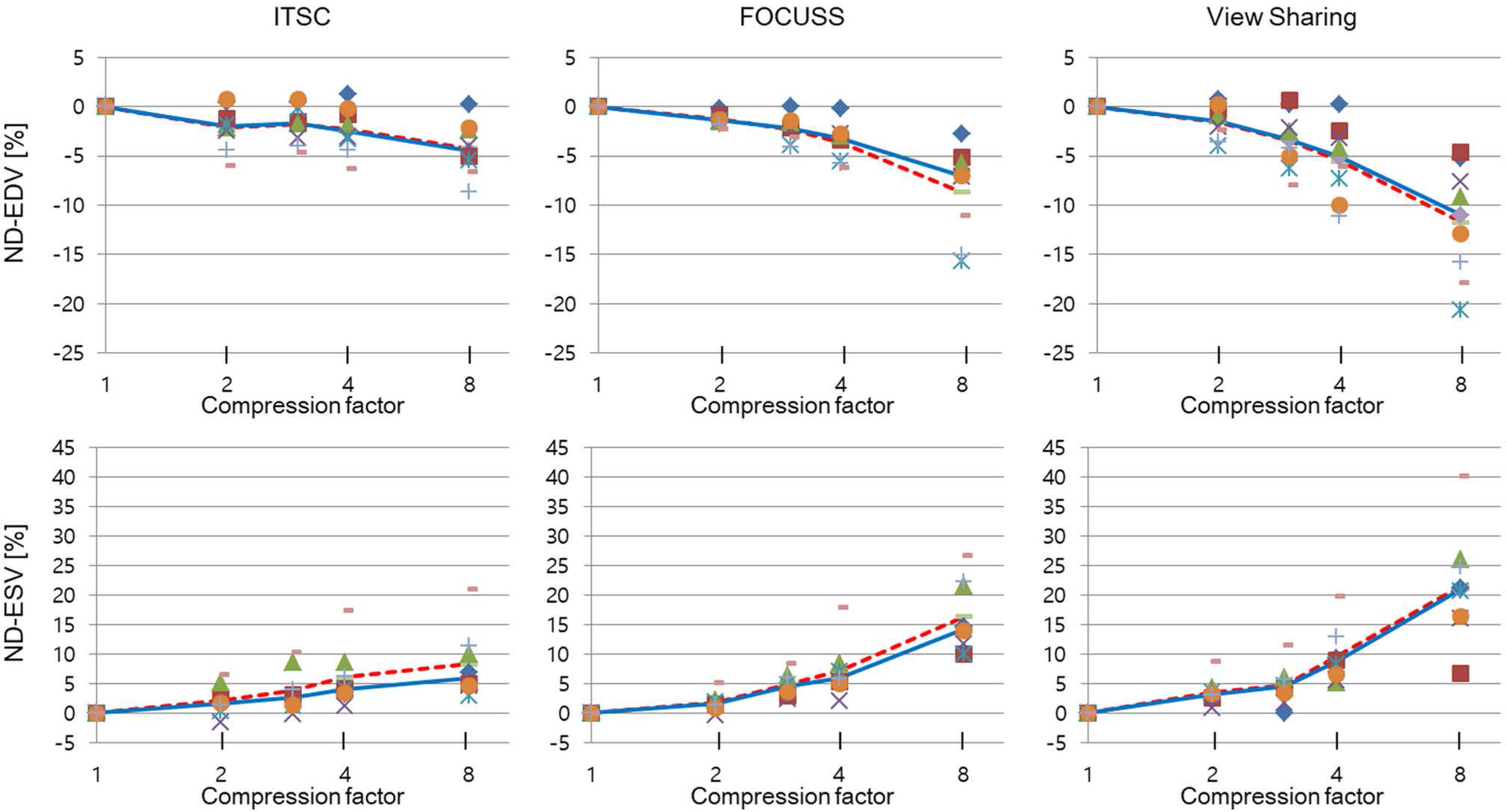
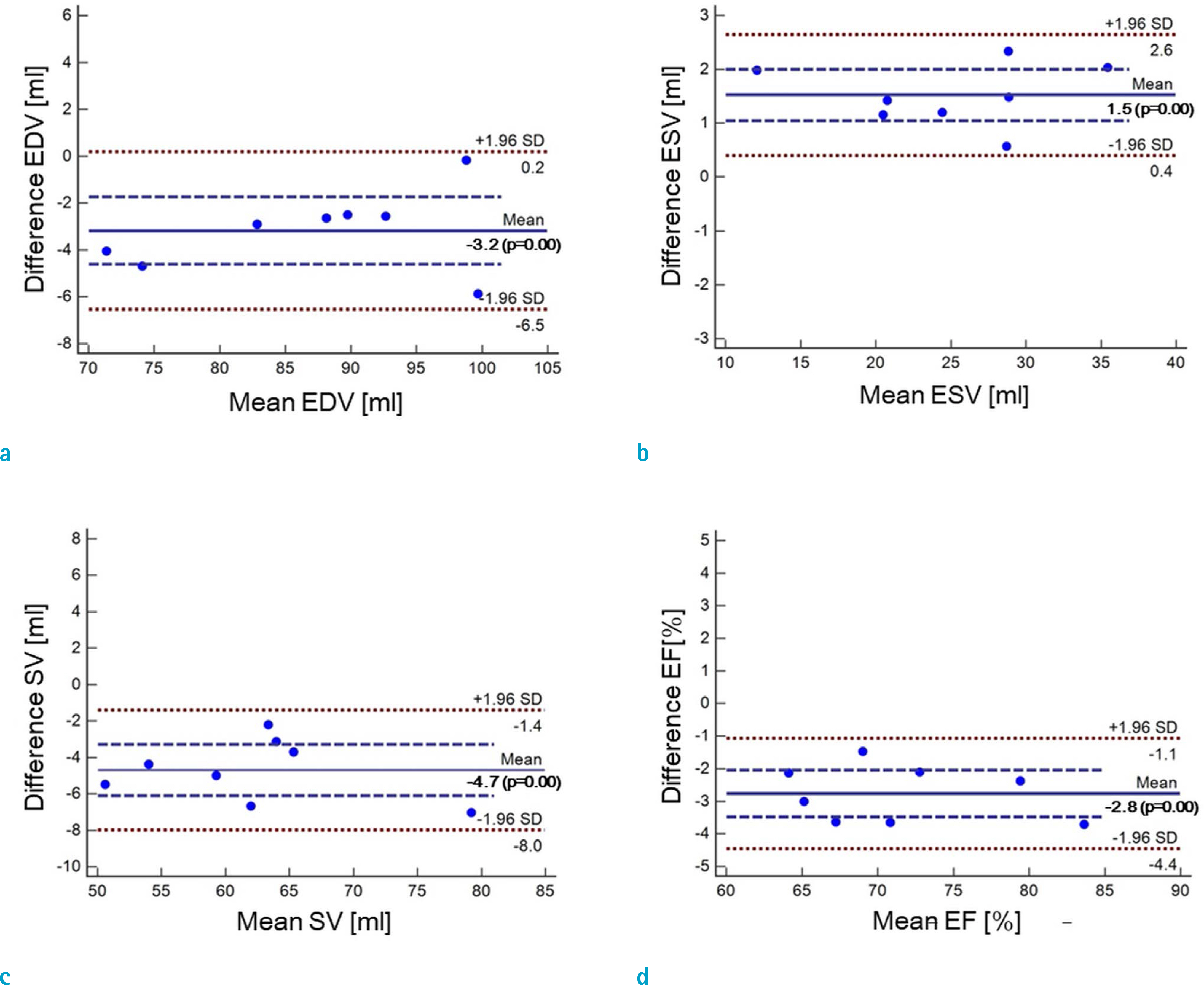
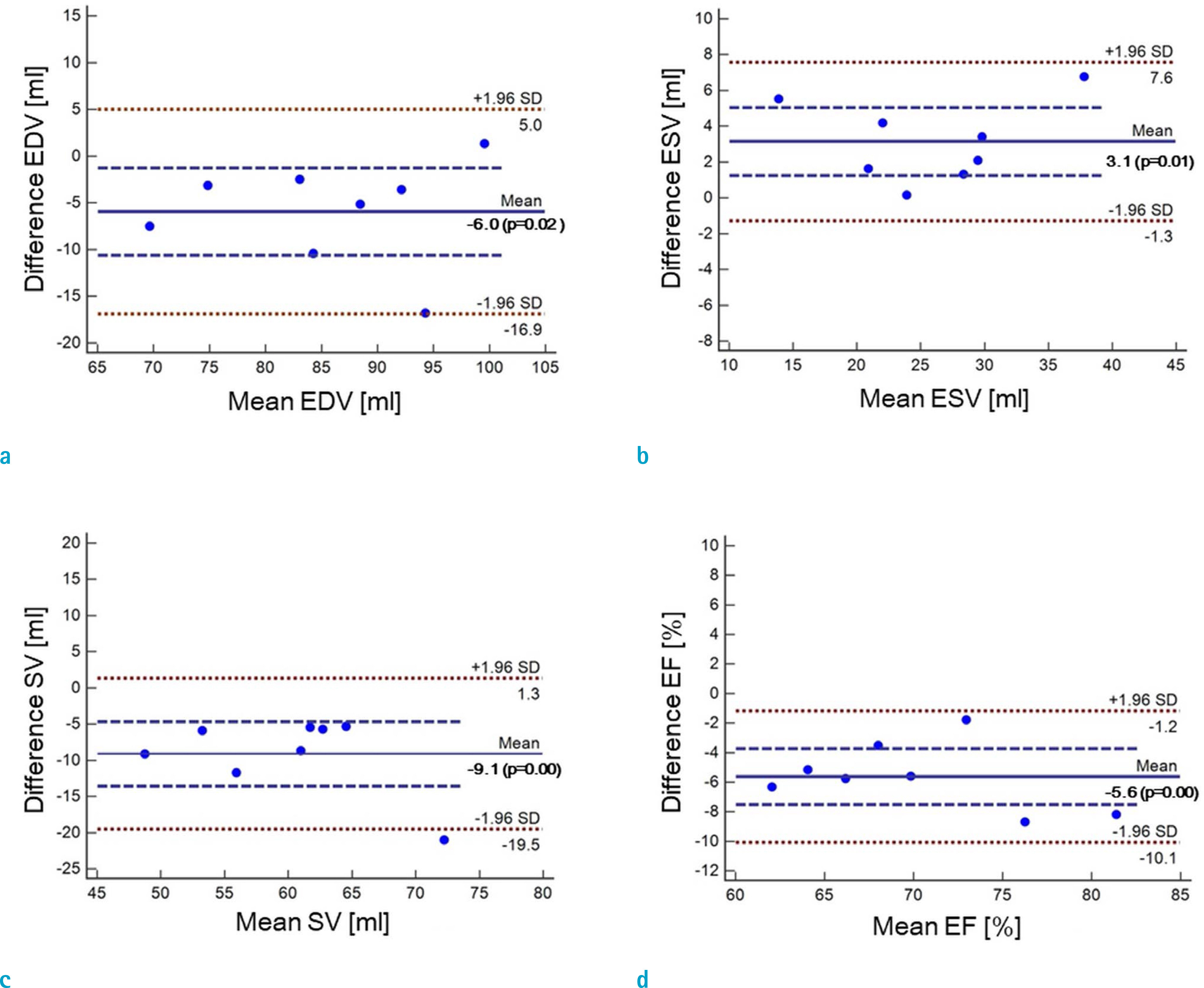
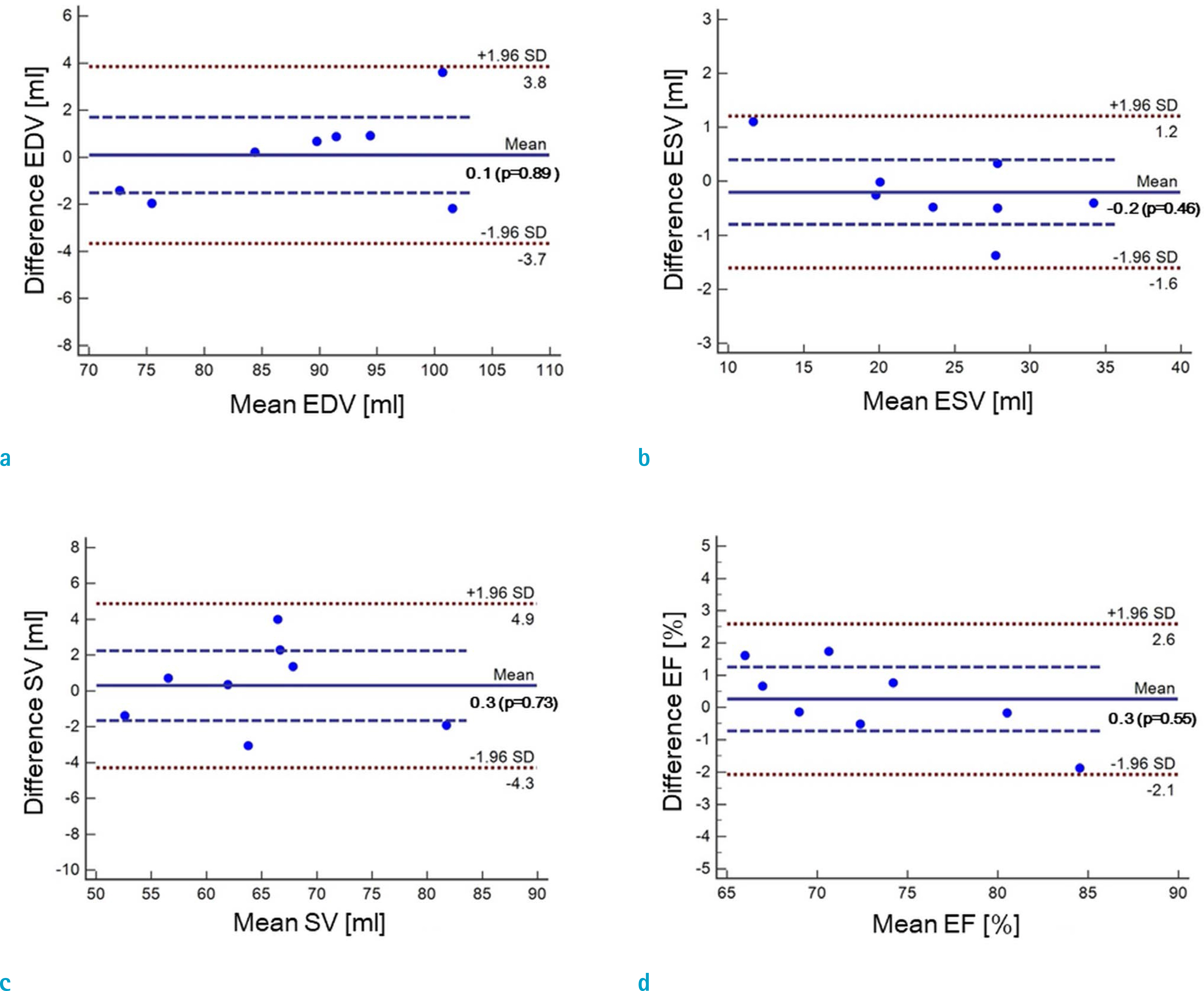
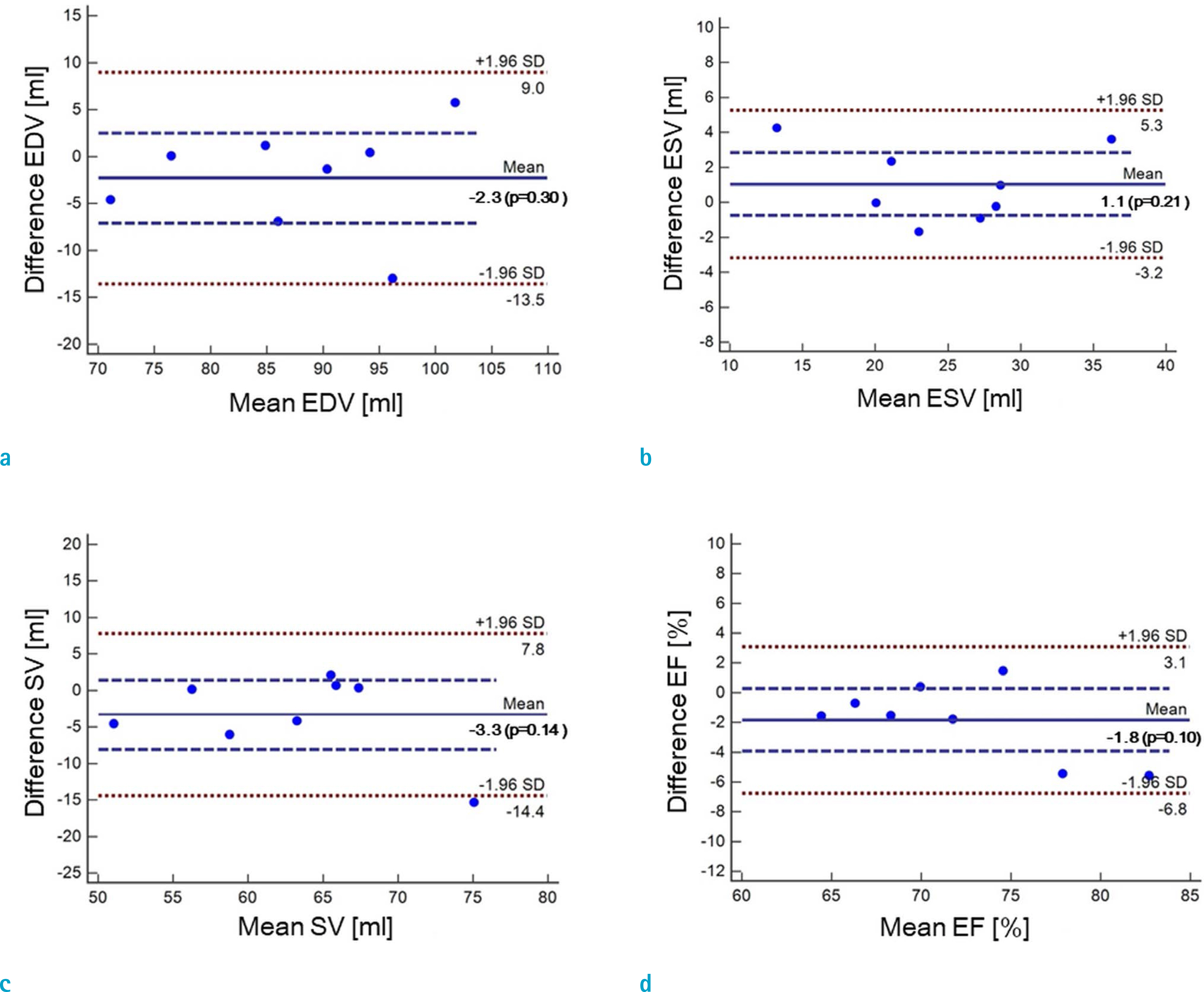
Cardiovascular cine images with short axis view, were obtained for 8 volunteers without CS. LVFs were assessed with subsampled data, with compression factors (CF) of 2, 3, 4, and 8. A semi-automatic segmentation program was used, for the assessment. The assessments by 3 CS methods (ITSC, FOCUSS, and view sharing (VS)), were compared to those without CS. Bland-Altman analysis and paired t-test were used, for comparison. In addition, real-time CS-cine imaging was also performed, with CF of 2, 3, 4, and 8 for the same volunteers. Assessments of LVF were similarly made, for CS data. A fixed compensation technique is suggested, to reduce the bias.
RESULTS
The assessment of LVF by CS-cine, includes bias and random noise. Bias appeared much larger than random noise. Median of end-diastolic volume (EDV) with CS-cine (ITSC or FOCUSS) appeared −1.4% to −7.1% smaller, compared to that of standard cine, depending on CF from (2 to 8). End-systolic volume (ESV) appeared +1.6% to +14.3% larger, stroke volume (SV), −2.4% to −16.4% smaller, and ejection fraction (EF), −1.1% to −9.2% smaller, with P < 0.05. Bias was reduced from −5.6% to −1.8% for EF, by compensation applied to real-time CS-cine (CF = 8).
CONCLUSION
Loss of temporal resolution by adopting missing data from nearby cardiac frames, causes an underestimation for EDV, and an overestimation for ESV, resulting in underestimations for SV and EF. The bias is not random. Thus it should be removed or reduced for better diagnosis. A fixed compensation is suggested, to reduce bias in the assessment of LVF.
MeSH Terms
Figure
Reference
-
References
1. Lamb HJ, Doornbos J, van der Velde EA, Kruit MC, Reiber JH, de Roos A. Echo planar MRI of the heart on a standard system: validation of measurements of left ventricular function and mass. J Comput Assist Tomogr. 1996; 20:942–949.
Article2. Epstein FH. MRI of left ventricular function. J Nucl Cardiol. 2007; 14:729–744.
Article3. Donoho DL. Compressed sensing. IEEE Trans Inf Theory. 2006; 52:1289–1306.
Article4. Baraniuk RG. Compressive sensing [lecture notes]. IEEE Signal Process Mag. 2007; 24:118–124.
Article5. Lustig M, Donoho D, Pauly JM. Sparse MRI: The application of compressed sensing for rapid MR imaging. Magn Reson Med. 2007; 58:1182–1195.
Article6. Candes EJ, Wakin MB. An introduction to compressive sampling. IEEE Signal Process Mag. 2008; 25:21–30.
Article7. Pruessmann KP, Weiger M, Scheidegger MB, Boesiger P. SENSE: sensitivity encoding for fast MRI. Magn Reson Med. 1999; 42:952–962.
Article8. Liang D, Liu B, Wang J, Ying L. Accelerating SENSE using compressed sensing. Magn Reson Med. 2009; 62:1574–1584.
Article9. Otazo R, Kim D, Axel L, Sodickson DK. Combination of compressed sensing and parallel imaging for highly accelerated first-pass cardiac perfusion MRI. Magn Reson Med. 2010; 64:767–776.
Article10. Feng L, Grimm R, Block KT, et al. Golden-angle radial sparse parallel MRI: combination of compressed sensing, parallel imaging, and golden-angle radial sampling for fast and flexible dynamic volumetric MRI. Magn Reson Med. 2014; 72:707–717.
Article11. Benkert T, Feng L, Sodickson DK, Chandarana H, Block KT. Free-breathing volumetric fat/water separation by combining radial sampling, compressed sensing, and parallel imaging. Magn Reson Med. 2017; 78:565–576.
Article12. Park J, Hong HJ, Yang YJ, Ahn CB. Fast cardiac CINE MRI by iterative truncation of small transformed coefficients. Investig Magn Reson Imaging. 2015; 19:19–30.
Article13. Aurigemma G, Reichek N, Schiebler M, Axel L. Evaluation of aortic regurgitation by cardiac cine magnetic resonance imaging: planar analysis and comparison to Doppler echocardiography. Cardiology. 1991; 78:340–347.
Article14. Rodevan O, Bjornerheim R, Ljosland M, Maehle J, Smith HJ, Ihlen H. Left atrial volumes assessed by three- and two-dimensional echocardiography compared to MRI estimates. Int J Card Imaging. 1999; 15:397–410.15. Bak SH, Kim SM, Park S, Kim M, Choe YH. Assessment of left ventricular function with single breathhold magnetic resonance cine imaging in patients with arrhythmia. Investig Magn Reson Imaging. 2017; 21:20–27.
Article16. Ngo TA, Lu Z, Carneiro G. Combining deep learning and level set for the automated segmentation of the left ventricle of the heart from cardiac cine magnetic resonance. Med Image Anal. 2017; 35:159–171.
Article17. White HD, Norris RM, Brown MA, Brandt PW, Whitlock RM, Wild CJ. Left ventricular end-systolic volume as the major determinant of survival after recovery from myocardial infarction. Circulation. 1987; 76:44–51.
Article18. Lorenz CH, Walker ES, Morgan VL, Klein SS, Graham TP Jr. Normal human right and left ventricular mass, systolic function, and gender differences by cine magnetic resonance imaging. J Cardiovasc Magn Reson. 1999; 1:7–21.
Article19. Wu E, Ortiz JT, Tejedor P, et al. Infarct size by contrast enhanced cardiac magnetic resonance is a stronger predictor of outcomes than left ventricular ejection fraction or end-systolic volume index: prospective cohort study. Heart. 2008; 94:730–736.
Article20. Muthurangu V, Lurz P, Critchely JD, Deanfield JE, Taylor AM, Hansen MS. Real-time assessment of right and left ventricular volumes and function in patients with congenital heart disease by using high spatiotemporal resolution radial k-t SENSE. Radiology. 2008; 248:782–791.
Article21. Miller S, Simonetti OP, Carr J, Kramer U, Finn JP. MR Imaging of the heart with cine true fast imaging with steady-state precession: influence of spatial and temporal resolutions on left ventricular functional parameters. Radiology. 2002; 223:263–269.
Article22. Hsiao A, Lustig M, Alley MT, et al. Rapid pediatric cardiac assessment of flow and ventricular volume with compressed sensing parallel imaging volumetric cine phase-contrast MRI. AJR Am J Roentgenol. 2012; 198:W250–259.
Article23. Goebel J, Nensa F, Schemuth HP, et al. Compressed sensing cine imaging with high spatial or high temporal resolution for analysis of left ventricular function. J Magn Reson Imaging. 2016; 44:366–374.
Article24. Vincenti G, Monney P, Chaptinel J, et al. Compressed sensing single-breathhold CMR for fast quantification of LV function, volumes, and mass. JACC Cardiovasc Imaging. 2014; 7:882–892.25. Bassett EC, Kholmovski EG, Wilson BD, et al. Evaluation of highly accelerated realtime cardiac cine MRI in tachycardia. NMR Biomed. 2014; 27:175–182.
Article26. Lin ACW, Strugnell W, Riley R, et al. Higher resolution cine imaging with compressed sensing for accelerated clinical left ventricular evaluation. J Magn Reson Imaging. 2017; 45:1693–1699.
Article27. Kido T, Kido T, Nakamura M, et al. Compressed sensing realtime cine cardiovascular magnetic resonance: accurate assessment of left ventricular function in a single-breathhold. J Cardiovasc Magn Reson. 2016; 18:50.
Article28. Bluemke DA, Boxerman JL, Atalar E, McVeigh ER. Segmented K-space cine breathhold cardiovascular MR imaging: Part 1. Principles and technique. AJR Am J Roentgenol. 1997; 169:395–400.29. Scheffler K, Lehnhardt S. Principles and applications of balanced SSFP techniques. Eur Radiol. 2003; 13:2409–2418.
Article30. Jung H, Sung K, Nayak KS, Kim EY, Ye JC. k-t FOCUSS: a general compressed sensing framework for high resolution dynamic MRI. Magn Reson Med. 2009; 61:103–116.
Article31. Markl M, Hennig J. Phase contrast MRI with improved temporal resolution by view sharing: k-space related velocity mapping properties. Magn Reson Imaging. 2001; 19:669–676.
Article32. Lang RM, Bierig M, Devereux RB, et al. Recommendations for chamber quantification: a report from the American Society of Echocardiography's Guidelines and Standards Committee and the Chamber Quantification Writing Group, developed in conjunction with the European Association of Echocardiography, a branch of the European Society of Cardiology. J Am Soc Echocardiogr. 2005; 18:1440–1463.
Article33. Lang RM, Badano LP, Mor-Avi V, et al. Recommendations for cardiac chamber quantification by echocardiography in adults: an update from the American Society of Echocardiography and the European Association of Cardiovascular Imaging. Eur Heart J Cardiovasc Imaging. 2015; 16:233–270.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Comparison of Left and Right Ventricular Volume and Cardiac Output by MRI and Echocardiography
- Fast Cardiac CINE MRI by Iterative Truncation of Small Transformed Coefficients
- Comparison between Echocardiography and Cardiac Cine-MRI : Left Ventricular Volume and Cardiac Output
- Comparison between Three-Dimensional Navigator-Gated Whole-Heart MRI and Two-Dimensional Cine MRI in Quantifying Ventricular Volumes
- Cardiac Strain Analysis Using Cine Magnetic Resonance Imaging and Computed Tomography