Investig Magn Reson Imaging.
2019 Jun;23(2):100-113. 10.13104/imri.2019.23.2.100.
Recent Update of Advanced Imaging for Diagnosis of Cardiac Sarcoidosis: Based on the Findings of Cardiac Magnetic Resonance Imaging and Positron Emission Tomography
- Affiliations
-
- 1Department of Radiology, Seoul St. Mary's Hospital, College of Medicine, The Catholic University of Korea, Seoul, Korea.
- 2Department of Nuclear Medicine, Seoul National University Bundang Hospital, Seongnam-si, Korea.
- 3Department of Radiology, Seoul National University Bundang Hospital, Seongnam-si, Korea. drejchun@hanmail.net
- KMID: 2452524
- DOI: http://doi.org/10.13104/imri.2019.23.2.100
Abstract
- Sarcoidosis is a multisystem disease characterized by noncaseating granulomas. Cardiac involvement is known to have poor prognosis because it can manifest as a serious condition such as the conduction abnormality, heart failure, ventricular arrhythmia, or sudden cardiac death. Although early diagnosis and early treatment is critical to improve patient prognosis, the diagnosis of CS is challenging in most cases. Diagnosis usually relies on endomyocardial biopsy (EMB), but its diagnostic yield is low due to the incidence of patchy myocardial involvement. Guidelines for the diagnosis of CS recommend a combination of clinical, electrocardiographic, and imaging findings from various modalities, if EMB cannot confirm the diagnosis. Especially, the role of advanced imaging such as cardiac magnetic resonance (CMR) imaging and positron emission tomography (PET), has shown to be important not only for the diagnosis, but also for monitoring treatment response and prognostication. CMR can evaluate cardiac function and fibrotic scar with good specificity. Late gadolinium enhancement (LGE) in CMR shows a distinctive enhancement pattern for each disease, which may be useful for differential diagnosis of CS from other similar diseases. Effectively, T1 or T2 mapping techniques can be also used for early recognition of CS. In the meantime, PET can detect and quantify metabolic activity and can be used to monitor treatment response. Recently, the use of a hybrid CMR-PET has introduced to allow identify patients with active CS with excellent co-localization and better diagnostic accuracy than CMR or PET alone. However, CS may show various findings with a wide spectrum, therefore, radiologists should consider the possible differential diagnosis of CS including myocarditis, dilated cardiomyopathy (DCM), hypertrophic cardiomyopathy, amyloidosis, and arrhythmogenic right ventricular cardiomyopathy. Radiologists should recognize the differences in various diseases that show the characteristics of mimicking CS, and try to get an accurate diagnosis of CS.
MeSH Terms
-
Amyloidosis
Arrhythmias, Cardiac
Arrhythmogenic Right Ventricular Dysplasia
Biopsy
Cardiomyopathy, Dilated
Cardiomyopathy, Hypertrophic
Cicatrix
Death, Sudden, Cardiac
Diagnosis*
Diagnosis, Differential
Early Diagnosis
Electrocardiography
Electrons*
Gadolinium
Granuloma
Heart Defects, Congenital
Humans
Incidence
Magnetic Resonance Imaging*
Myocarditis
Positron-Emission Tomography*
Prognosis
Sarcoidosis*
Sensitivity and Specificity
Gadolinium
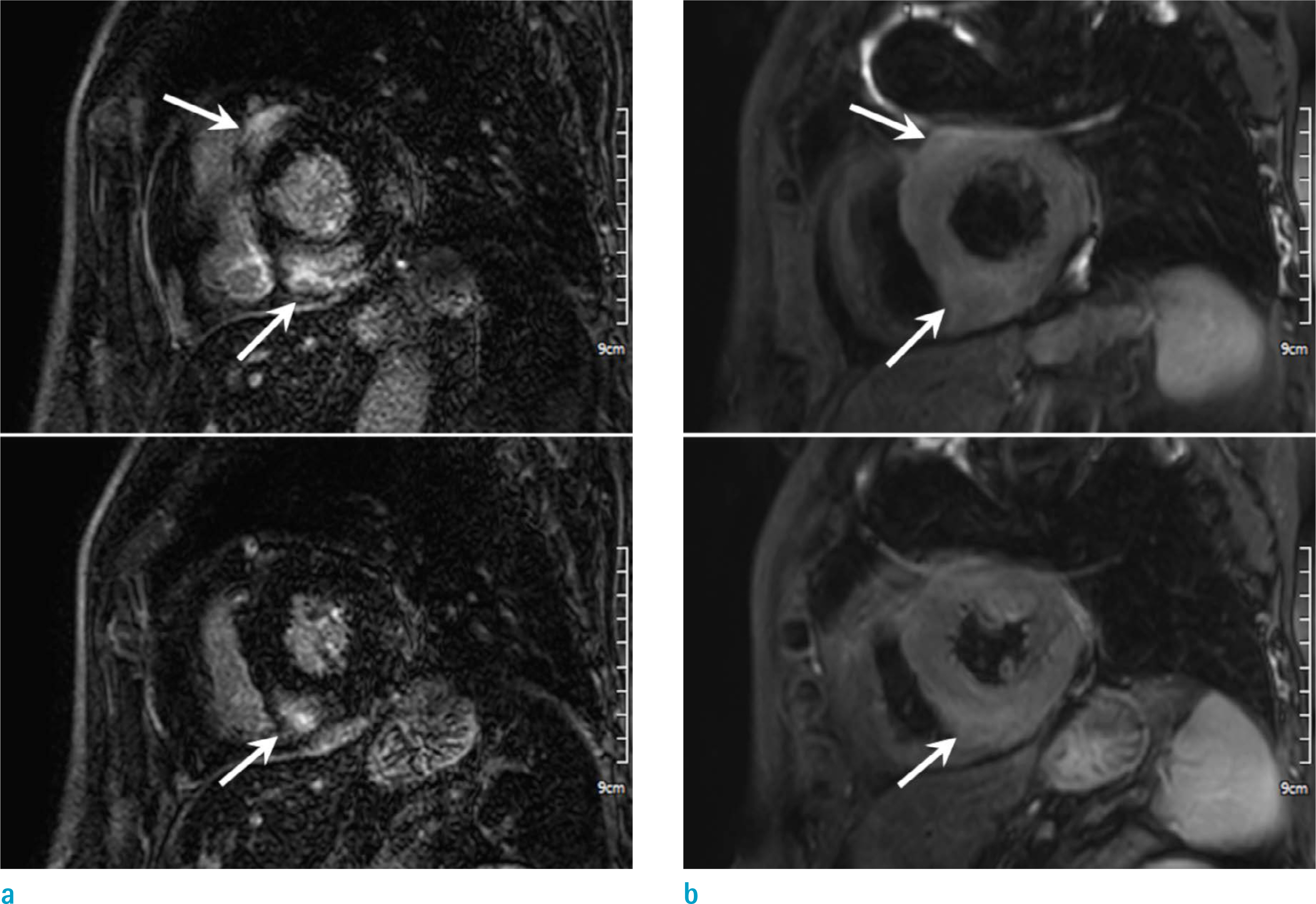
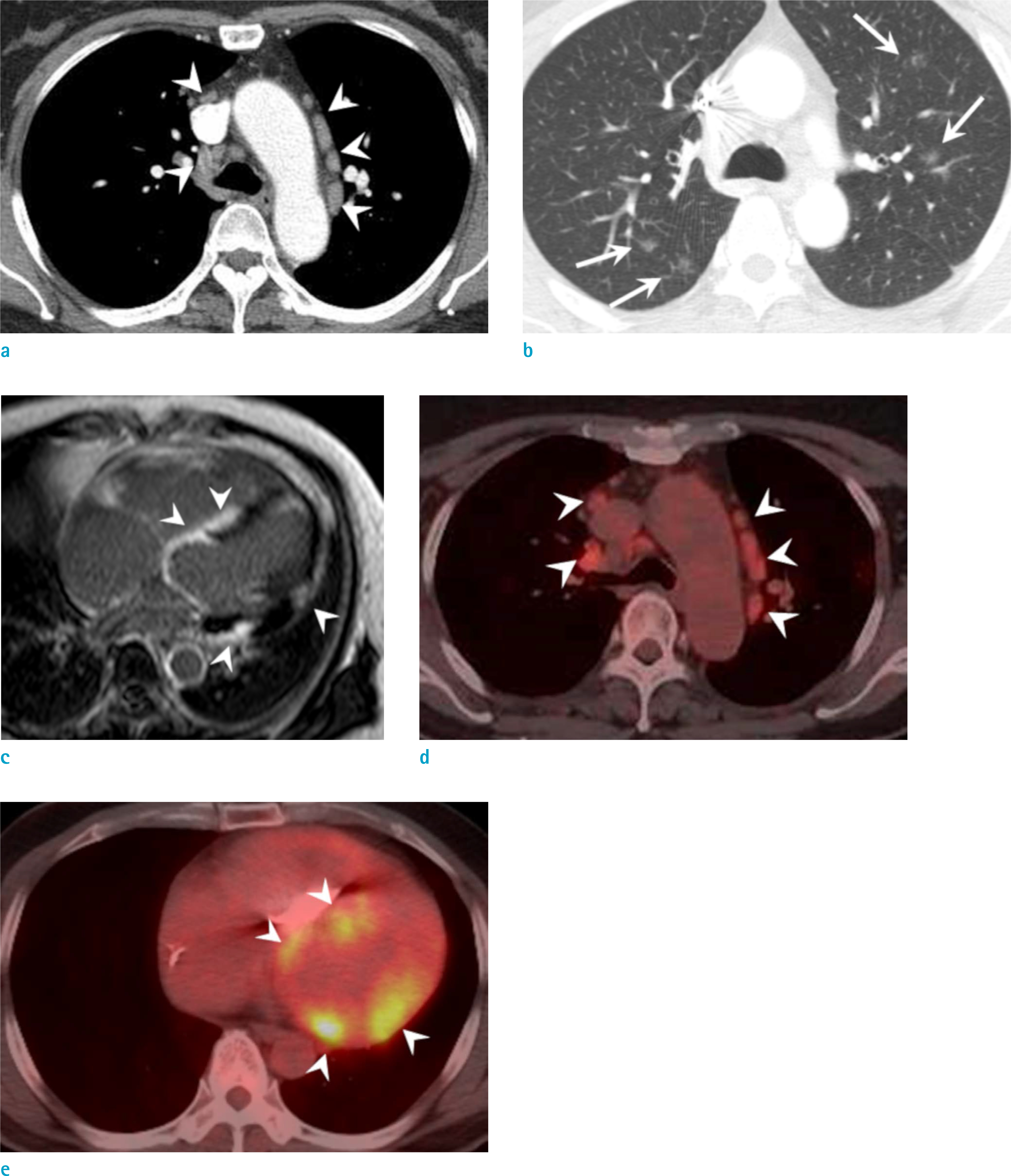
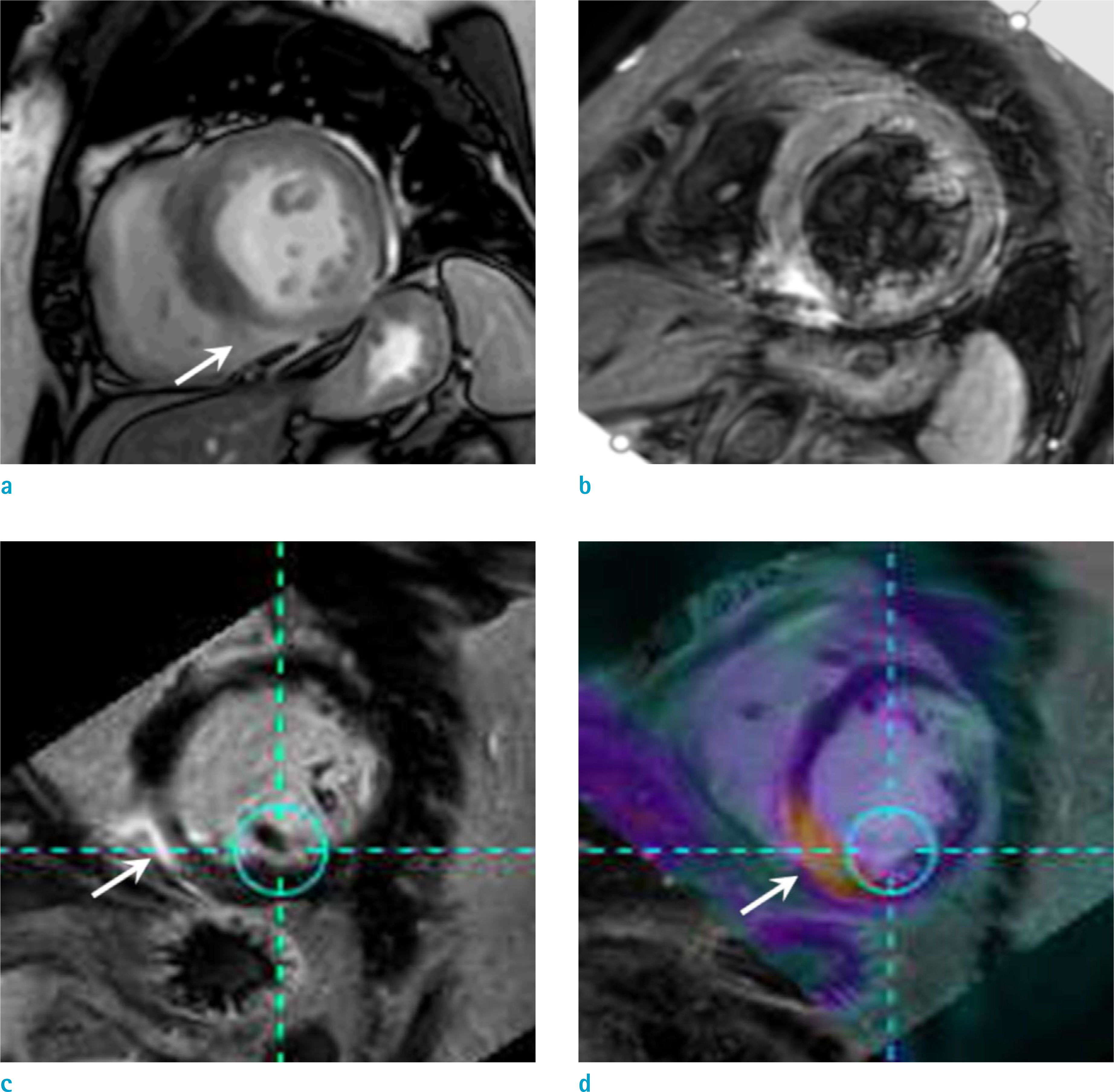
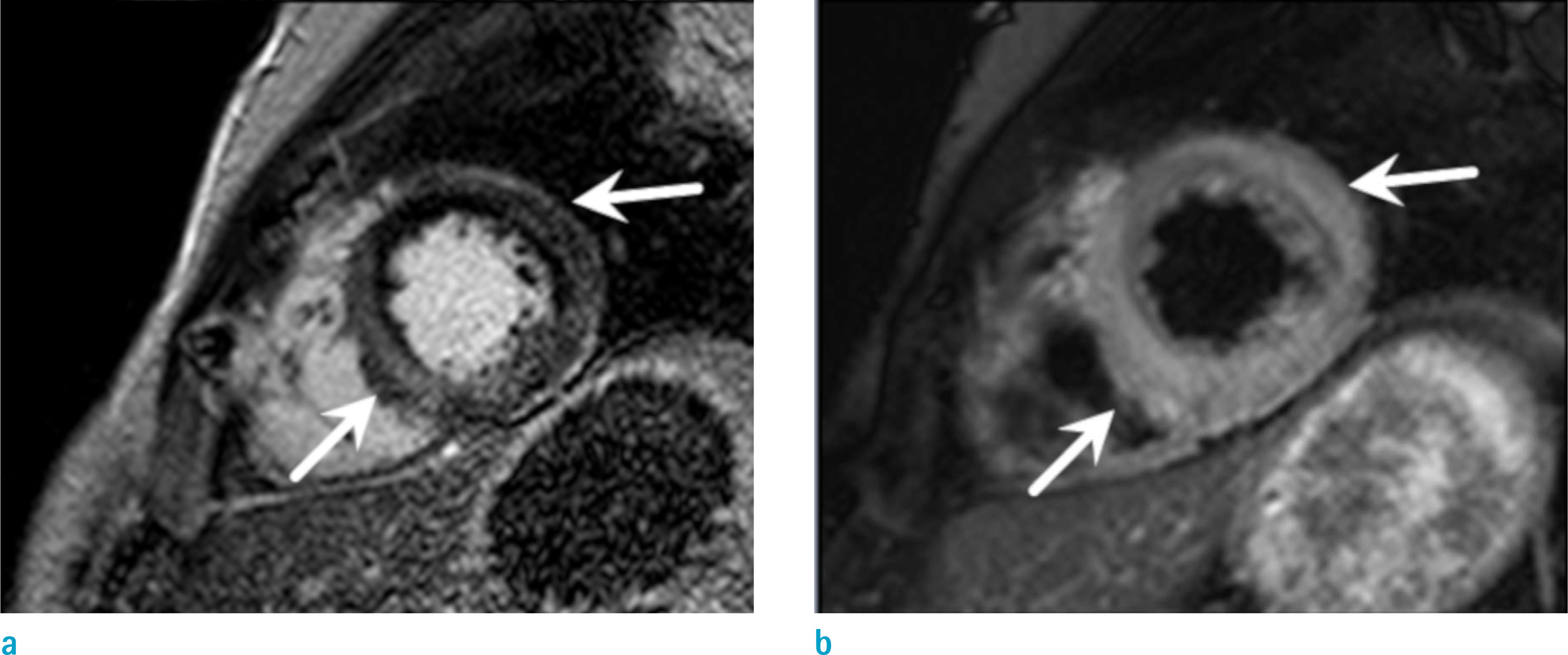
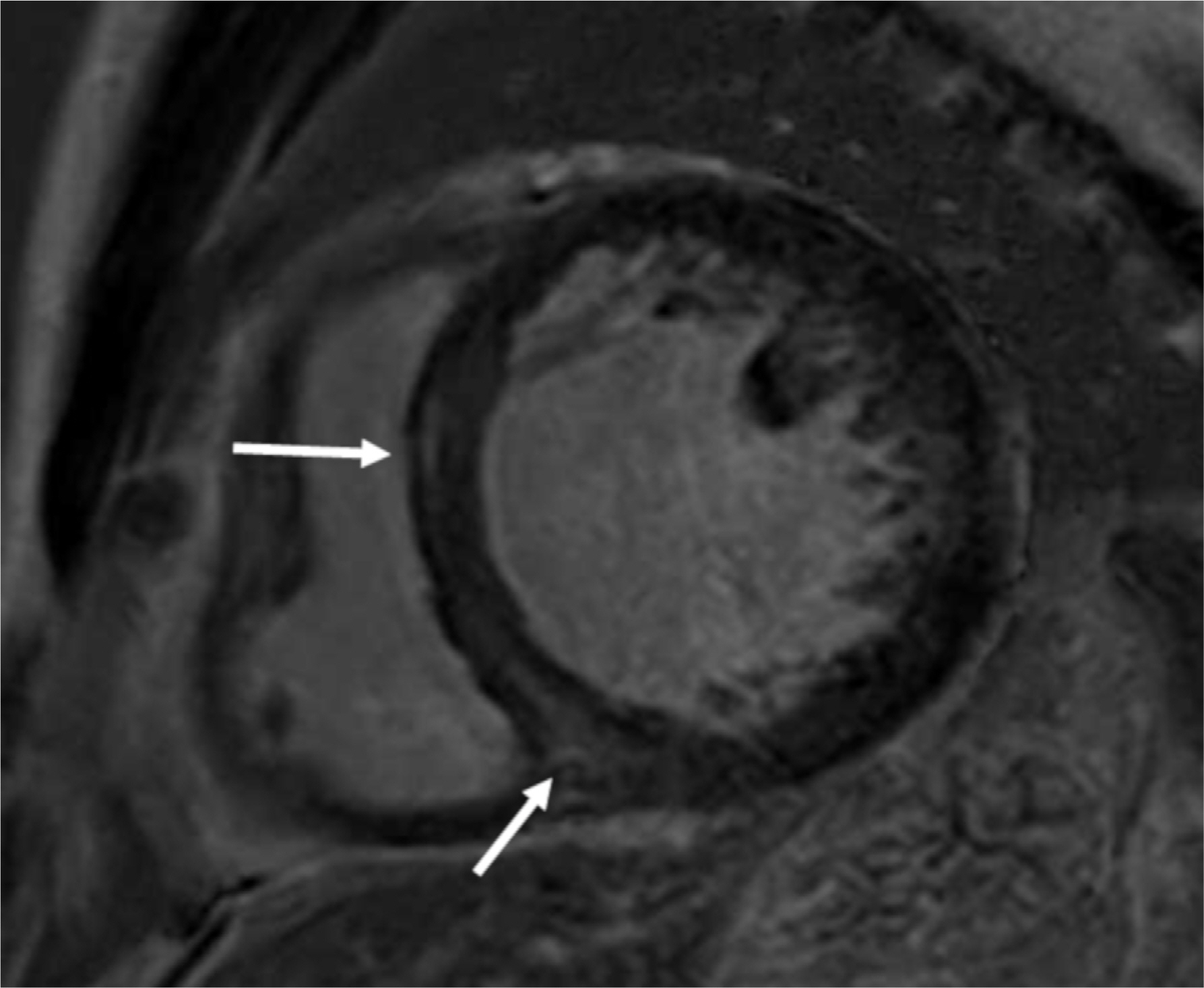
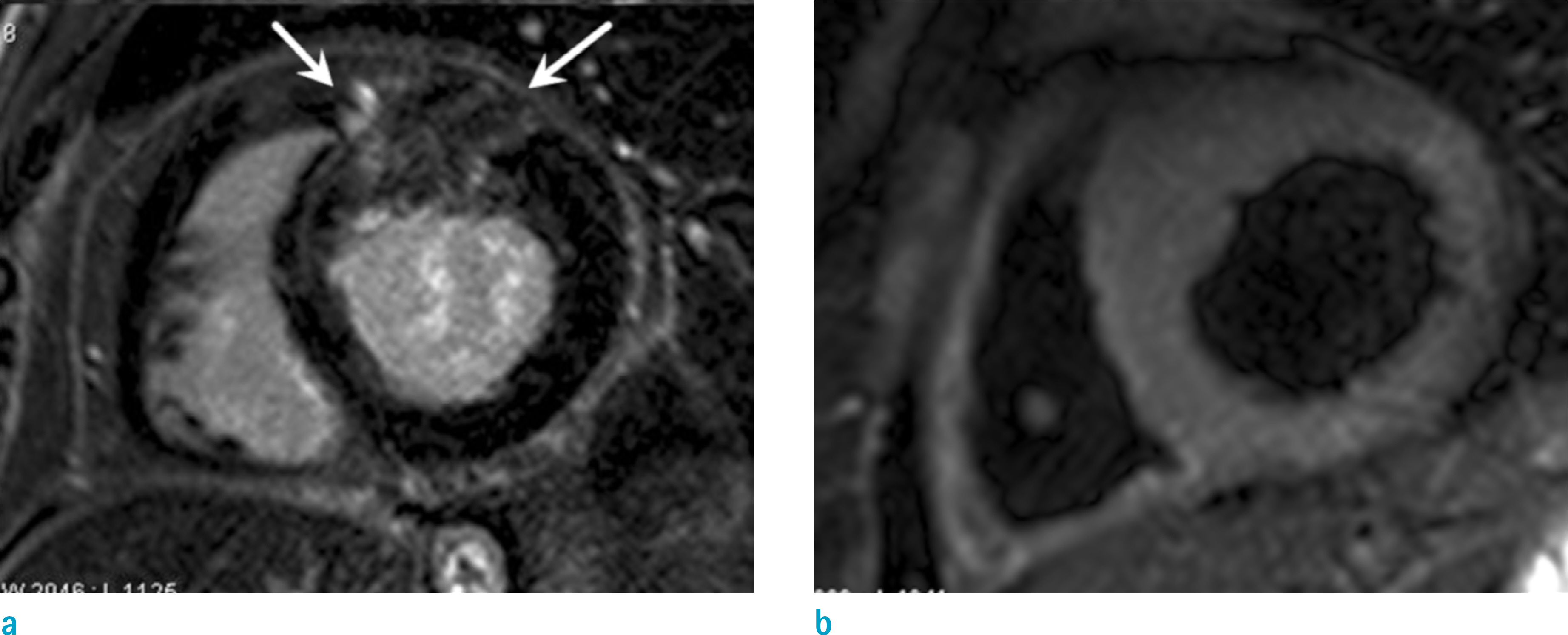
Figure
Reference
-
References
1. Statement on sarcoidosis. Joint Statement of the American Thoracic Society (ATS), the European Respiratory Society (ERS) and the World Association of Sarcoidosis and Other Granulomatous Disorders (WASOG) adopted by the ATS Board of Directors and by the ERS Executive Committee, February 1999. Am J Respir Crit Care Med. 1999; 160:736–755.2. Schatka I, Bengel FM. Advanced imaging of cardiac sarcoidosis. J Nucl Med. 2014; 55:99–106.
Article3. Silverman KJ, Hutchins GM, Bulkley BH. Cardiac sarcoid: a clinicopathologic study of 84 unselected patients with systemic sarcoidosis. Circulation. 1978; 58:1204–1211.
Article4. Lynch JP 3rd, Hwang J, Bradfield J, Fishbein M, Shivkumar K, Tung R. Cardiac involvement in sarcoidosis: evolving concepts in diagnosis and treatment. Semin Respir Crit Care Med. 2014; 35:372–390.
Article5. Yazaki Y, Isobe M, Hiroe M, et al. Prognostic determinants of longterm survival in Japanese patients with cardiac sarcoidosis treated with prednisone. Am J Cardiol. 2001; 88:1006–1010.
Article6. Ardehali H, Howard DL, Hariri A, et al. A positive endomyocardial biopsy result for sarcoid is associated with poor prognosis in patients with initially unexplained cardiomyopathy. Am Heart J. 2005; 150:459–463.
Article7. Hillerdal G, Nou E, Osterman K, Schmekel B. Sarcoidosis: epidemiology and prognosis. A 15-year European study. Am Rev Respir Dis. 1984; 130:29–32.8. Morimoto T, Azuma A, Abe S, et al. Epidemiology of sarcoidosis in Japan. Eur Respir J. 2008; 31:372–379.
Article9. Varron L, Cottin V, Schott AM, Broussolle C, Seve P. Late-onset sarcoidosis: a comparative study. Medicine (Baltimore). 2012; 91:137–143.10. Perry A, Vuitch F. Causes of death in patients with sarcoidosis. A morphologic study of 38 autopsies with clinicopathologic correlations. Arch Pathol Lab Med. 1995; 119:167–172.11. Iwai K, Tachibana T, Takemura T, Matsui Y, Kitaichi M, Kawabata Y. Pathological studies on sarcoidosis autopsy. I. Epidemiological features of 320 cases in Japan. Acta Pathol Jpn. 1993; 43:372–376.
Article12. Mehta D, Lubitz SA, Frankel Z, et al. Cardiac involvement in patients with sarcoidosis: diagnostic and prognostic value of outpatient testing. Chest. 2008; 133:1426–1435.13. Rosen Y. Pathology of sarcoidosis. Semin Respir Crit Care Med. 2007; 28:36–52.
Article14. Uemura A, Morimoto S, Hiramitsu S, Kato Y, Ito T, Hishida H. Histologic diagnostic rate of cardiac sarcoidosis: evaluation of endomyocardial biopsies. Am Heart J. 1999; 138:299–302.
Article15. Tavora F, Cresswell N, Li L, Ripple M, Solomon C, Burke A. Comparison of necropsy findings in patients with sarcoidosis dying suddenly from cardiac sarcoidosis versus dying suddenly from other causes. Am J Cardiol. 2009; 104:571–577.
Article16. Birnie DH, Nery PB, Ha AC, Beanlands RS. Cardiac sarcoidosis. J Am Coll Cardiol. 2016; 68:411–421.
Article17. Youssef G, Beanlands RS, Birnie DH, Nery PB. Cardiac sarcoidosis: applications of imaging in diagnosis and directing treatment. Heart. 2011; 97:2078–2087.
Article18. Sekhri V, Sanal S, Delorenzo LJ, Aronow WS, Maguire GP. Cardiac sarcoidosis: a comprehensive review. Arch Med Sci. 2011; 7:546–554.
Article19. Hiraga HHM, Iwai K. Guidelines for diagnosis of cardiac sarcoidosis: study report on diffuse pulmonary diseases (in Japanese). Tokyo: The Japanese Ministry of Health and Welfare. 1993. 23–24.20. Soejima K, Yada H. The workup and management of patients with apparent or subclinical cardiac sarcoidosis: with emphasis on the associated heart rhythm abnormalities. J Cardiovasc Electrophysiol. 2009; 20:578–583.
Article21. Youssef G, Leung E, Mylonas I, et al. The use of 18F-FDG PET in the diagnosis of cardiac sarcoidosis: a systematic review and metaanalysis including the Ontario experience. J Nucl Med. 2012; 53:241–248.
Article22. Blankstein R, Osborne M, Naya M, et al. Cardiac positron emission tomography enhances prognostic assessments of patients with suspected cardiac sarcoidosis. J Am Coll Cardiol. 2014; 63:329–336.
Article23. Kouranos V, Wells AU, Sharma R, Underwood SR, Wechalekar K. Advances in radionuclide imaging of cardiac sarcoidosis. Br Med Bull. 2015; 115:151–163.
Article24. Birnie DH, Sauer WH, Bogun F, et al. HRS expert consensus statement on the diagnosis and management of arrhythmias associated with cardiac sarcoidosis. Heart Rhythm. 2014; 11:1305–1323.
Article25. Hulten E, Aslam S, Osborne M, Abbasi S, Bittencourt MS, Blankstein R. Cardiac sarcoidosis-state of the art review. Cardiovasc Diagn Ther. 2016; 6:50–63.26. Jeudy J, Burke AP, White CS, Kramer GB, Frazier AA. Cardiac sarcoidosis: the challenge of radiologic-pathologic correlation: from the radiologic pathology archives. Radiographics. 2015; 35:657–679.27. Puntmann VO, Isted A, Hinojar R, Foote L, Carr-White G, Nagel E. T1 and T2 mapping in recognition of early cardiac involvement in systemic sarcoidosis. Radiology. 2017; 285:63–72.
Article28. Kouranos V, Tzelepis GE, Rapti A, et al. Complementary role of CMR to conventional screening in the diagnosis and prognosis of cardiac sarcoidosis. JACC Cardiovasc Imaging. 2017; 10:1437–1447.
Article29. Patel MR, Cawley PJ, Heitner JF, et al. Detection of myocardial damage in patients with sarcoidosis. Circulation. 2009; 120:1969–1977.
Article30. Greulich S, Deluigi CC, Gloekler S, et al. CMR imaging predicts death and other adverse events in suspected cardiac sarcoidosis. JACC Cardiovasc Imaging. 2013; 6:501–511.
Article31. Ise T, Hasegawa T, Morita Y, et al. Extensive late gadolinium enhancement on cardiovascular magnetic resonance predicts adverse outcomes and lack of improvement in LV function after steroid therapy in cardiac sarcoidosis. Heart. 2014; 100:1165–1172.
Article32. Crouser ED, Ono C, Tran T, He X, Raman SV. Improved detection of cardiac sarcoidosis using magnetic resonance with myocardial T2 mapping. Am J Respir Crit Care Med. 2014; 189:109–112.33. Perez IE, Garcia MJ, Taub CC. Multimodality imaging in cardiac sarcoidosis: is there a winner? Curr Cardiol Rev. 2016; 12:3–11.34. Orii M, Imanishi T, Akasaka T. Assessment of cardiac sarcoidosis with advanced imaging modalities. Biomed Res Int. 2014; 2014:897956.
Article35. Roberts WC, McAllister HA Jr, Ferrans VJ. Sarcoidosis of the heart. A clinicopathologic study of 35 necropsy patients (group 1) and review of 78 previously described necropsy patients (group 11). Am J Med. 1977; 63:86–108.36. Greulich S, Kitterer D, Latus J, et al. Comprehensive cardiovascular magnetic resonance assessment in patients with sarcoidosis and preserved left ventricular ejection fraction. Circ Cardiovasc Imaging. 2016; 9:e005022.
Article37. Ferreira VM, Piechnik SK. Seeing beyond the obvious: subclinical cardiac sarcoidosis revealed by cardiovascular magnetic resonance mapping. Circ Cardiovasc Imaging. 2016; 9:e005592.38. Hinojar R, Foote L, Arroyo Ucar E, et al. Native T1 in discrimination of acute and convalescent stages in patients with clinical diagnosis of myocarditis: a proposed diagnostic algorithm using CMR. JACC Cardiovasc Imaging. 2015; 8:37–46.39. Manoushagian SJ, Lakhter V, Patil PV. Multimodality imaging in the diagnosis and management of cardiac sarcoidosis. J Nucl Cardiol. 2017; 24:29–33.
Article40. Langah R, Spicer K, Gebregziabher M, Gordon L. Effectiveness of prolonged fasting 18f-FDG PET-CT in the detection of cardiac sarcoidosis. J Nucl Cardiol. 2009; 16:801–810.
Article41. Ishimaru S, Tsujino I, Takei T, et al. Focal uptake on 18F-fluoro-2-deoxyglucose positron emission tomography images indicates cardiac involvement of sarcoidosis. Eur Heart J. 2005; 26:1538–1543.
Article42. Harisankar CN, Mittal BR, Agrawal KL, Abrar ML, Bhattacharya A. Utility of high fat and low carbohydrate diet in suppressing myocardial FDG uptake. J Nucl Cardiol. 2011; 18:926–936.
Article43. Betensky BP, Tschabrunn CM, Zado ES, et al. Longterm follow-up of patients with cardiac sarcoidosis and implantable cardioverter-defibrillators. Heart Rhythm. 2012; 9:884–891.
Article44. Ahmadian A, Brogan A, Berman J, et al. Quantitative interpretation of FDG PET/CT with myocardial perfusion imaging increases diagnostic information in the evaluation of cardiac sarcoidosis. J Nucl Cardiol. 2014; 21:925–939.
Article45. Gormsen LC, Haraldsen A, Kramer S, Dias AH, Kim WY, Borghammer P. A dual tracer (68)Ga-DOTANOC PET/CT and (18)F-FDG PET/CT pilot study for detection of cardiac sarcoidosis. EJNMMI Res. 2016; 6:52.
Article46. Manabe O, Hirata K, Shozo O, et al. (18)F-fluoromisonidazole (FMISO) PET may have the potential to detect cardiac sarcoidosis. J Nucl Cardiol. 2017; 24:329–331.
Article47. Ohira H, Tsujino I, Ishimaru S, et al. Myocardial imaging with 18F-fluoro-2-deoxyglucose positron emission tomography and magnetic resonance imaging in sarcoidosis. Eur J Nucl Med Mol Imaging. 2008; 35:933–941.
Article48. Dweck MR, Abgral R, Trivieri MG, et al. Hybrid magnetic resonance imaging and positron emission tomography with fluorodeoxyglucose to diagnose active cardiac sarcoidosis. JACC Cardiovasc Imaging. 2018; 11:94–107.49. Wicks EC, Menezes LJ, Barnes A, et al. Diagnostic accuracy and prognostic value of simultaneous hybrid 18F-fluorodeoxyglucose positron emission tomography/magnetic resonance imaging in cardiac sarcoidosis. Eur Heart J Cardiovasc Imaging. 2018; 19:757–767.
Article50. Ishida Y, Yoshinaga K, Miyagawa M, et al. Recommendations for (18)F-fluorodeoxyglucose positron emission tomography imaging for cardiac sarcoidosis: Japanese Society of Nuclear Cardiology recommendations. Ann Nucl Med. 2014; 28:393–403.
Article51. Friedrich MG, Sechtem U, Schulz-Menger J, et al. Cardiovascular magnetic resonance in myocarditis: a JACC white paper. J Am Coll Cardiol. 2009; 53:1475–1487.
Article52. Yilmaz A, Ferreira V, Klingel K, Kandolf R, Neubauer S, Sechtem U. Role of cardiovascular magnetic resonance imaging (CMR) in the diagnosis of acute and chronic myocarditis. Heart Fail Rev. 2013; 18:747–760.
Article53. Yazaki Y, Isobe M, Hiramitsu S, et al. Comparison of clinical features and prognosis of cardiac sarcoidosis and idiopathic dilated cardiomyopathy. Am J Cardiol. 1998; 82:537–540.
Article54. Vignaux O. Cardiac sarcoidosis: spectrum of MRI features. AJR Am J Roentgenol. 2005; 184:249–254.
Article55. Moraes GL, Higgins CB, Ordovas KG. Delayed enhancement magnetic resonance imaging in nonischemic myocardial disease. J Thorac Imaging. 2013; 28:84–92. quiz 93–95.
Article56. Marcus FI, McKenna WJ, Sherrill D, et al. Diagnosis of arrhythmogenic right ventricular cardiomyopathy/dysplasia: proposed modification of the task force criteria. Circulation. 2010; 121:1533–1541.57. Steckman DA, Schneider PM, Schuller JL, et al. Utility of cardiac magnetic resonance imaging to differentiate cardiac sarcoidosis from arrhythmogenic right ventricular cardiomyopathy. Am J Cardiol. 2012; 110:575–579.
Article58. Bartlett ML, Bacharach SL, Voipio-Pulkki LM, Dilsizian V. Artifactual inhomogeneities in myocardial PET and SPECT scans in normal subjects. J Nucl Med. 1995; 36:188–195.59. Tahara N, Tahara A, Nitta Y, et al. Heterogeneous myocardial FDG uptake and the disease activity in cardiac sarcoidosis. JACC Cardiovasc Imaging. 2010; 3:1219–1228.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- The Role of Multimodality Imaging in Cardiac Sarcoidosis
- Multimodality Imaging Can Help to Doubt, Diagnose and Follow-Up Cardiac Mass
- Management of Arrhythmias Associated with Cardiac Sarcoidosis
- Radiologic Evaluation and Differential Diagnosis of Gallbladder Cancer
- A Case of Sacroiliitis Diagnosed with Positron-Emission Tomography with Normal Magnetic Resonance Imaging Finding