Obstet Gynecol Sci.
2019 Jul;62(4):285-289. 10.5468/ogs.2019.62.4.285.
Long-term survival after intraperitoneal chemotherapy with paclitaxel-cisplatin for recurrent primary peritoneal cancer resistant to multiple lines of intravenous chemotherapy
- Affiliations
-
- 1Department of Obstetrics and Gynecology, Seoul National University Hospital, Seoul, Korea.
- 2Department of Obstetrics and Gynecology, Seoul National University Bundang Hospital, Seongnam, Korea. kidong.kim.md@gmail.com
- 3Department of Pathology, Seoul National University Bundang Hospital, Seongnam, Korea.
- KMID: 2451659
- DOI: http://doi.org/10.5468/ogs.2019.62.4.285
Abstract
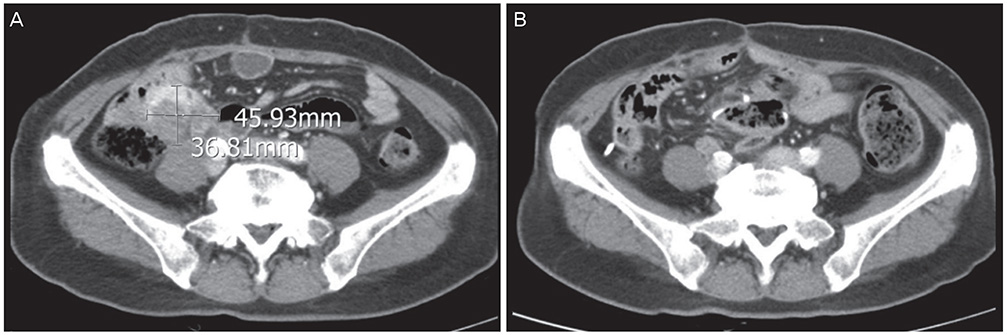
- The long-term survival of heavily pretreated patients with primary peritoneal cancer (PPC) is uncommon. Here, we report on a patient with PPC refractory to multiple lines of intravenous chemotherapy, namely, a combined regimen of paclitaxel and carboplatin, and single regimens of topotecan, docetaxel, cisplatin, and gemcitabine. However, after intraperitoneal (IP) chemotherapy with paclitaxel-cisplatin, the patient's condition improved, and she has been progression-free for more than 4 years. Interestingly, before the IP chemotherapy, the recurrences were limited to the peritoneal cavity. These results suggest that IP recurrence might be a predictor of a good response to IP chemotherapy.
Keyword
MeSH Terms
Figure
Reference
-
1. Jung KW, Won YJ, Kong HJ, Lee ES. Prediction of cancer incidence and mortality in Korea, 2018. Cancer Res Treat. 2018; 50:317–323.
Article2. Statistics Korea. Cancer registry statistics [Internet]. Daejeon: Statistics Korea;2018. cited 2018 Feb 8. Available from: http://kosis.kr.3. Armstrong DK. Relapsed ovarian cancer: challenges and management strategies for a chronic disease. Oncologist. 2002; 7:Suppl 5. 20–28.
Article4. Vermorken JB. Intraperitoneal chemotherapy in advanced ovarian cancer: recognition at last. Ann Oncol. 2006; 17:Suppl 10. x241–x246.
Article5. Morgan RJ Jr, Armstrong DK, Alvarez RD, Bakkum-Gamez JN, Behbakht K, Chen LM, et al. Ovarian cancer, version 1.2016, NCCN Clinical Practice Guidelines in Oncology. J Natl Compr Canc Netw. 2016; 14:1134–1163.6. Pujade-Lauraine E, Hilpert F, Weber B, Reuss A, Poveda A, Kristensen G, et al. Bevacizumab combined with chemotherapy for platinum-resistant recurrent ovarian cancer: the AURELIA open-label randomized phase III trial. J Clin Oncol. 2014; 32:1302–1308.
Article7. Alberts DS, Liu PY, Hannigan EV, O'Toole R, Williams SD, Young JA, et al. Intraperitoneal cisplatin plus intravenous cyclophosphamide versus intravenous cisplatin plus intravenous cyclophosphamide for stage III ovarian cancer. N Engl J Med. 1996; 335:1950–1955.
Article8. Armstrong DK, Bundy B, Wenzel L, Huang HQ, Baergen R, Lele S, et al. Intraperitoneal cisplatin and paclitaxel in ovarian cancer. N Engl J Med. 2006; 354:34–43.
Article9. Markman M, Bundy BN, Alberts DS, Fowler JM, Clark-Pearson DL, Carson LF, et al. Phase III trial of standard-dose intravenous cisplatin plus paclitaxel versus moderately high-dose carboplatin followed by intravenous paclitaxel and intraperitoneal cisplatin in small-volume stage III ovarian carcinoma: an intergroup study of the Gynecologic Oncology Group, Southwestern Oncology Group, and Eastern Cooperative Oncology Group. J Clin Oncol. 2001; 19:1001–1007.
Article10. Yen MS, Juang CM, Lai CR, Chao GC, Ng HT, Yuan CC. Intraperitoneal cisplatin-based chemotherapy vs. intravenous cisplatin-based chemotherapy for stage III optimally cytoreduced epithelial ovarian cancer. Int J Gynaecol Obstet. 2001; 72:55–60.
Article11. Walker J, Brady MF, DiSilvestro PA, Fujiwara K, Alberts D, Zheng W, et al. A phase III trial of bevacizumab with IV versus IP chemotherapy for ovarian, fallopian tube, and peritoneal carcinoma: an NRG Oncology Study. Gynecol Oncol. 2016; 141 :Suppl 1. 208.
Article12. Vermorken JB. The role of intraperitoneal chemotherapy in epithelial ovarian cancer. Int J Gynecol Cancer. 2000; 10:26–32.
Article13. Lesnock JL, Darcy KM, Tian C, Deloia JA, Thrall MM, Zahn C, et al. BRCA1 expression and improved survival in ovarian cancer patients treated with intraperitoneal cisplatin and paclitaxel: a Gynecologic Oncology Group Study. Br J Cancer. 2013; 108:1231–1237.
Article14. Seagle BL, Eng KH, Yeh JY, Dandapani M, Schiller E, Samuelson R, et al. Discovery of candidate tumor biomarkers for treatment with intraperitoneal chemotherapy for ovarian cancer. Sci Rep. 2016; 6:21591.
Article15. Esselen KM, Rodriguez N, Growdon W, Krasner C, Horowitz NS, Campos S. Patterns of recurrence in advanced epithelial ovarian, fallopian tube and peritoneal cancers treated with intraperitoneal chemotherapy. Gynecol Oncol. 2012; 127:51–54.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Effect of Radical Removal of Primary and Metastatic Lesions in Gastric Cancer with Peritoneal Seeding
- Treatment of Peritoneal Carcinomatosis from Colorectal Cancer
- Long-Term Survival Analysis of Intraperitoneal versus Intravenous Chemotherapy for Primary Ovarian Cancer and Comparison between Carboplatin- and Cisplatin-based Intraperitoneal Chemotherapy
- A role of Cyclosporine A that suppresses multi-drug resistance (MDR) in the secondary chemotherapy drug resistant cell line of ovarian cancer
- Clinical Evaluation for the Effect of Intraoperative Intraperitoneal Chemotherapy in Advanced Gastric Cancer