Neonatal Med.
2019 May;26(2):102-110. 10.5385/nm.2019.26.2.102.
Effect of Delayed Elevation of Thyrotropin on Feeding Intolerance in Very Low Birth Weight Infants
- Affiliations
-
- 1Department of Pediatrics, Kosin University Gospel Hospital, Kosin University College of Medicine, Busan, Korea. agasoa@kosin.ac.kr
- 2Department of Pediatrics, Inje University Haeundae Paik Hospital, Inje University College of Medicine, Busan, Korea.
- KMID: 2451608
- DOI: http://doi.org/10.5385/nm.2019.26.2.102
Abstract
- PURPOSE
We investigated the effect of delayed elevation of thyrotropin (TSH) (deTSH) on gastrointestinal motility in very low birth weight infants (VLBWI).
METHODS
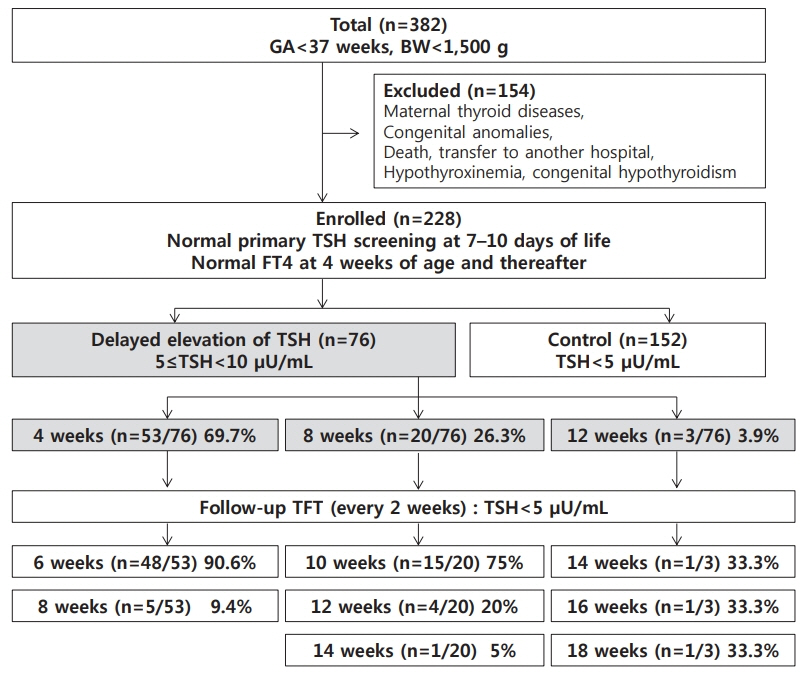
This study retrospectively investigated 228 premature VLBWI aged ≥4 weeks with normal neonatal TSH screening test results and free serum thyroxine levels. Infants with serum TSH levels ranging from 5 to 10 µIU/mL were categorized as the deTSH group (n=76), when TSH was measured at 4 (n=53), 8 (n=20), or 12 (n=3) weeks of age. Serum TSH levels in the control group (n=152) were <5 µIU/mL. Multivariate logistic regression analysis was used to determine the risk factors for the development of deTSH. Covariance analysis was used to analyze the relationship between deTSH and gastrointestinal motility.
RESULTS
The mean gestational age and birth weight were 29.11±2.25 weeks and 1,157.4±218.0 g, respectively. Risk factors affecting deTSH were dopamine administration (odds ratio [OR], 8.71; 95% confidence interval [CI], 1.80 to 42.05; P=0.007) and operation time (OR, 6.95; 95% CI, 1.43 to 33.75; P=0.016) when the cumulative operating time was ≥1 hour. The mean±standard deviation (SD) duration of a nil per os (NPO) status was significantly higher in the deTSH (99.57±134.99 hours) than in the control group (37.25±59.02 hours) (P from analysis of covariance [ANCOVA]=0.001). The mean±SD duration (33.84±22.34 days) of total parenteral nutrition (TPN) was considerably longer in the deTSH group than in the control group (27.68±13.08 days) (P from ANCOVA=0.003).
CONCLUSION
Clinicians must consider deTSH in VLBWI showing feeding intolerance with a prolonged NPO and TPN status.
MeSH Terms
Figure
Reference
-
1. Gruters A, Biebermann H, Krude H. Neonatal thyroid disorders. Horm Res. 2003; 59 Suppl 1:24–9.2. Smith L. Updated AAP guidelines on newborn screening and therapy for congenital hypothyroidism. Am Fam Physician. 2007; 76:439–44.3. Kim JD. Hypothyroidism. Korean J Pediatr. 2005; 48:779–805.4. Fisher DA. Thyroid function and dysfunction in premature infants. Pediatr Endocrinol Rev. 2007; 4:317–28.5. Vigone MC, Caiulo S, Di Frenna M, Ghirardello S, Corbetta C, Mosca F, et al. Evolution of thyroid function in preterm infants detected by screening for congenital hypothyroidism. J Pediatr. 2014; 164:1296–302.6. American Academy of Pediatrics, Rose SR; Section on Endocrinology and Committee on Genetics; American Thyroid Association, Brown RS; Public Health Committee; Lawson Wilkins Pediatric Endocrine Society, Foley T, et al. Update of newborn screening and therapy for congenital hypothyroidism. Pediatrics. 2006; 117:2290–303.7. Leger J, Olivieri A, Donaldson M, Torresani T, Krude H, van Vliet G, et al. European Society for Paediatric Endocrinology consensus guidelines on screening, diagnosis, and management of congenital hypothyroidism. Horm Res Paediatr. 2014; 81:80–103.8. Divall SA, Wondisford FE. TRH testing in its infancy. J Clin Endocrinol Metab. 2008; 93:378–9.9. Murphy N, Hume R, van Toor H, Matthews TG, Ogston SA, Wu SY, et al. The hypothalamic-pituitary-thyroid axis in preterm infants: changes in the first 24 hours of postnatal life. J Clin Endocrinol Metab. 2004; 89:2824–31.10. Chung HR, Shin CH, Yang SW, Choi CW, Kim BI, Kim EK, et al. High incidence of thyroid dysfunction in preterm infants. J Korean Med Sci. 2009; 24:627–31.11. Fisher DA. Hypothyroxinemia in premature infants: is thyroxine treatment necessary? Thyroid. 1999; 9:715–20.12. Frank JE, Faix JE, Hermos RJ, Mullaney DM, Rojan DA, Mitchell ML, et al. Thyroid function in very low birth weight infants: effects on neonatal hypothyroidism screening. J Pediatr. 1996; 128:548–54.13. Smerdely P, Lim A, Boyages SC, Waite K, Wu D, Roberts V, et al. Topical iodine-containing antiseptics and neonatal hypothyroidism in very-low-birthweight infants. Lancet. 1989; 2:661–4.14. Choi EK, Lee HS, Lee EH, Kim SY, Lee BK, Jung YH, et al. Comparison of enteral feeding in early neonatal period in very low birthweight infants with hypothyroidism. Korean J Perinatol. 2015; 26:46–52.15. Battaglia FC, Lubchenco LO. A practical classification of newborn infants by weight and gestational age. J Pediatr. 1967; 71:159–63.16. Charafeddine L, D'Angio CT, Phelps DL. Atypical chronic lung disease patterns in neonates. Pediatrics. 1999; 103:759–65.17. Jobe AH, Bancalari E. Bronchopulmonary dysplasia. Am J Respir Crit Care Med. 2001; 163:1723–9.18. Walsh MC, Kliegman RM. Necrotizing enterocolitis: treatment based on staging criteria. Pediatr Clin North Am. 1986; 33:179–201.19. Volpe JJ. Intracranial hemorrhage: germinal matrix-intraventricular hemorrhage of the premature infant. In : Volpe JJ, editor. Neurology of the newborn. 4th ed. Philadelphia: WB Saunders Co.;2001. p. 428–93.20. Shepard TH. Onset of function in the human fetal thyroid: biochemical and radioautographic studies from organ culture. J Clin Endocrinol Metab. 1967; 27:945–58.21. Contempre B, Jauniaux E, Calvo R, Jurkovic D, Campbell S, de Escobar GM. Detection of thyroid hormones in human embryonic cavities during the first trimester of pregnancy. J Clin Endocrinol Metab. 1993; 77:1719–22.22. Morreale de Escobar G, Obregon MJ, Escobar del Rey F. Role of thyroid hormone during early brain development. Eur J Endocrinol. 2004; 151 Suppl 3:U25–37.23. Rastogi MV, LaFranchi SH. Congenital hypothyroidism. Orphanet J Rare Dis. 2010; 5:17.24. Lee JM, Choi TY, Lee DW, Lee DH. Recheck rate, recall rate and reference range of the neonatal screening test for congenital hypothyroidism. J Clin Pathol Qual Control. 2001; 23:215–20.25. Kim EY. Thyroid disorders in premature and sick newborns. Neonatal Med. 2015; 22:117–23.26. Fisher DA. Thyroid system immaturities in very low birth weight premature infants. Semin Perinatol. 2008; 32:387–97.27. Kim SY, Han MY, Lee KH. Thyroid function in preterm infants with respiratory distress syndrome and bronchopulmonary dysplasia. J Korean Soc Neonatal. 2001; 8:94–102.28. Hong KB, Park JY, Chang YP, Yu J. Thyroid dysfunction in premature infants. Korean J Pediatr. 2009; 52:991–8.29. Guy VV, Johnny D. Disorders of the thyroid in the newborns and infant. In : Sperling MA, editor. Pediatric endocrinology. 4th ed. Philadelphia: Elsevier Saunders;2014. p. 186–208.30. Filippi L, Pezzati M, Poggi C, Rossi S, Cecchi A, Santoro C. Dopaine versus dobutamine in very low birthweight infants: endocrine effects. Arch Dis Child Fetal Neonatal Ed. 2007; 92:F367–71.31. Filippi L, Pezzati M, Cecchi A, Poggi C. Dopamine infusion: a possible cause of undiagnosed congenital hypothyroidism in preterm infants. Pediatr Crit Care Med. 2006; 7:249–51.32. Zung A, Bier Palmon R, Golan A, Troitzky M, Eventov-Friedman S, Marom R, et al. Risk factors for the development of delayed TSH elevation in neonatal intensive care unit newborns. J Clin Endocrinol Metab. 2017; 102:3050–5.33. Uchiyama A, Watanabe H, Nakanishi H, Totsu S. Small for gestational age is a risk factor for the development of delayed thyrotropin elevation in infants weighing less than 2000 g. Clin Endocrinol (Oxf). 2018; 89:431–6.34. Marks SD. Nonthyroidal illness syndrome in children. Endocrine. 2009; 36:355–67.35. Hamblin PS, Dyer SA, Mohr VS, Le Grand BA, Lim CF, Tuxen DV, et al. Relationship between thyrotropin and thyroxine changes during recovery from severe hypothyroxinemia of critical illness. J Clin Endocrinol Metab. 1986; 62:717–22.36. Bacci V, Schussler GC, Kaplan TB. The relationship between serum triiodothyronine and thyrotropin during systemic illness. J Clin Endocrinol Metab. 1982; 54:1229–35.37. Kaluarachchi DC, Colaizy TT, Pesce LM, Tansey M, Klein JM. Congenital hypothyroidism with delayed thyroid-stimulating hormone elevation in premature infants born at less than 30 weeks gestation. J Perinatol. 2017; 37:277–82.38. Fanaro S. Feeding intolerance in the preterm infant. Early Hum Dev. 2013; 89 Suppl 2:S13–20.39. Jadcherla SR, Kliegman RM. Studies of feeding intolerance in very low birth weight infants: definition and significance. Pediatrics. 2002; 109:516–7.40. Sellappan B, Chakraborty M, Cherian S. Congenital hypothyroidism presenting as pseudo-obstruction in preterm infants. BMJ Case Rep. 2014; 2014:bcr2013201082.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Efficacy of Erythromycin and Metoclopramide in Neonates with Feeding Intolerance
- Clinical Review of Small Bowel Series in Forty Six Preterm Infants with Feeding Intolerance
- Feeding Introlerance Due to Allergic Enterocolitis in Very Low Birth Weight Infants
- Clinical and Statistical Observation for Low Birth Weight Infants
- Factors Influencing the Time to Full Enteral Feeding in Very Low Birth Weight Infants