J Clin Neurol.
2019 Jan;15(1):90-96. 10.3988/jcn.2019.15.1.90.
Development and Validation of the Cluster Headache Screening Questionnaire
- Affiliations
-
- 1Department of Neurology, Kangbuk Samsung Hospital, Sungkyunkwan University School of Medicine, Seoul, Korea.
- 2Department of Neurology, Dongtan Sacred Heart Hospital, Hallym University College of Medicine, Hwaseong, Korea. dowonc@naver.com
- 3Department of Neurology, Eulji Hospital, Eulji University, Seoul, Korea.
- 4Department of Neurology, Gyeonsang National University College of Medicine, Jinju, Korea.
- 5Department of Neurology, Neuroscience Center, Samsung Medical Center, Sungkyunkwan University School of Medicine, Seoul, Korea.
- 6Department of Neurology, Presbyterian Medical Center, Jeonju, Korea.
- 7Department of Neurology, Uijeongbu St. Mary's Hospital, The Catholic University of Korea, Uijeongbu, Korea.
- 8Department of Neurology, Bundang Jesaeng General Hospital, Daejin Medical Center, Seongnam, Korea.
- 9Department of Neurology, Korea University College of Medicine, Seoul, Korea.
- 10Department of Neurology, Ewha Womans University College of Medicine, Seoul, Korea.
- 11Department of Clinical Research Design and Evaluation, SAIHST, Sungkyunkwan University, Seoul, Korea.
- 12Center for Clinical Epidemiology, Samsung Medical Center, Sungkyunkwan University School of Medicine, Seoul, Korea.
- KMID: 2451150
- DOI: http://doi.org/10.3988/jcn.2019.15.1.90
Abstract
- BACKGROUND AND PURPOSE
Cluster headache (CH) is frequently either not diagnosed or the diagnosis is delayed. We addressed this issue by developing the self-administered Cluster Headache Screening Questionnaire (CHSQ).
METHODS
Experts selected items from the diagnostic criteria of CH and the characteristics of migraine. The questionnaire was administered to first-visit headache patients at nine headache clinics. The finally developed CHSQ included items based on the differences in responses between CH and non-CH patients, and the accuracy and reliability of the scoring model were assessed.
RESULTS
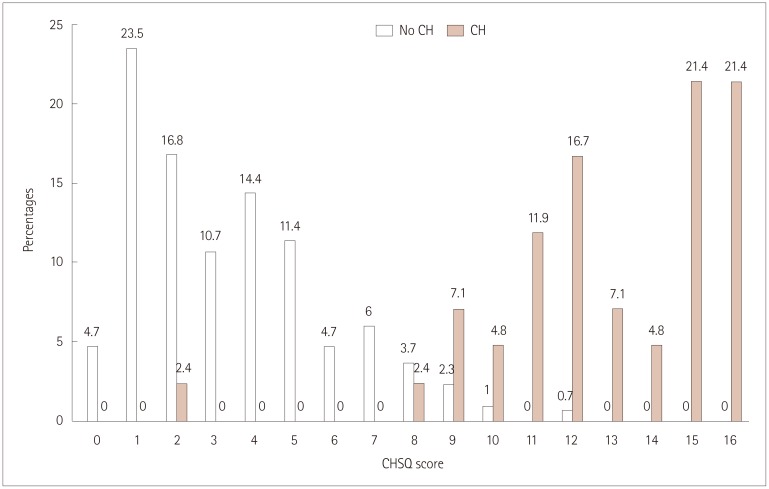
Forty-two patients with CH, 207 migraineurs, 73 with tension-type headache, and 18 with primary stabbing headache were enrolled. The CHSQ item were scored as follows: 3 points for ipsilateral eye symptoms, agitation, and duration; 2 points for clustering patterns; and 1 point for the male sex, unilateral pain, disability, ipsilateral nasal symptoms, and frequency. The total score of the CHSQ ranged from 0 to 16. The mean score was higher in patients with CH than in non-CH patients (12.9 vs. 3.4, p < 0.001). At a cutoff score of >8 points, the CHSQ had a sensitivity, specificity, positive predictive value, and negative predictive value of 95.2%, 96%, 76.9%, and 99.3%, respectively.
CONCLUSIONS
The CHSQ is a reliable screening tool for the rapid identification of CH.
Keyword
MeSH Terms
Figure
Reference
-
1. Goadsby PJ. Pathophysiology of cluster headache: a trigeminal autonomic cephalgia. Lancet Neurol. 2002; 1:251–257. PMID: 12849458.
Article2. Jensen RM, Lyngberg A, Jensen RH. Burden of cluster headache. Cephalalgia. 2007; 27:535–541. PMID: 17459083.
Article3. Abu Bakar N, Torkamani M, Tanprawate S, Lambru G, Matharu M, Jahanshahi M. The development and validation of the Cluster Headache Quality of life scale (CHQ). J Headache Pain. 2016; 17:79. PMID: 27596922.
Article4. Fischera M, Marziniak M, Gralow I, Evers S. The incidence and prevalence of cluster headache: a meta-analysis of population-based studies. Cephalalgia. 2008; 28:614–618. PMID: 18422717.
Article5. Ekbom K, Svensson DA, Träff H, Waldenlind E. Age at onset and sex ratio in cluster headache: observations over three decades. Cephalalgia. 2002; 22:94–100. PMID: 11972575.
Article6. Moon HS, Park JW, Lee KS, Chung CS, Kim BK, Kim JM, et al. Clinical features of cluster headache patients in Korea. J Korean Med Sci. 2017; 32:502–506. PMID: 28145655.
Article7. Gaul C, Finken J, Biermann J, Mostardt S, Diener HC, Müller O, et al. Treatment costs and indirect costs of cluster headache: a health economics analysis. Cephalalgia. 2011; 31:1664–1672. PMID: 21994114.
Article8. Klapper JA, Klapper A, Voss T. The misdiagnosis of cluster headache: a nonclinic, population-based, internet survey. Headache. 2000; 40:730–735. PMID: 11091291.
Article9. van Vliet JA, Eekers PJ, Haan J, Ferrari MD. Dutch RUSSH Study Group. Features involved in the diagnostic delay of cluster headache. J Neurol Neurosurg Psychiatry. 2003; 74:1123–1125. PMID: 12876249.
Article10. Viana M, Tassorelli C, Allena M, Nappi G, Sjaastad O, Antonaci F. Diagnostic and therapeutic errors in trigeminal autonomic cephalalgias and hemicrania continua: a systematic review. J Headache Pain. 2013; 14:14. PMID: 23565739.
Article11. Robbins MS, Starling AJ, Pringsheim TM, Becker WJ, Schwedt TJ. Treatment of cluster headache: the American Headache Society evidence-based guidelines. Headache. 2016; 56:1093–1106. PMID: 27432623.
Article12. Leroux E, Valade D, Taifas I, Vicaut E, Chagnon M, Roos C, et al. Suboccipital steroid injections for transitional treatment of patients with more than two cluster headache attacks per day: a randomised, double-blind, placebo-controlled trial. Lancet Neurol. 2011; 10:891–897. PMID: 21903477.
Article13. Wilbrink LA, Weller CM, Cheung C, Stijnen T, Haan J, Ferrari MD, et al. Stepwise web-based questionnaires for diagnosing cluster headache: LUCA and QATCH. Cephalalgia. 2013; 33:924–931. PMID: 23624341.
Article14. Dousset V, Laporte A, Legoff M, Traineau MH, Dartigues JF, Brochet B. Validation of a brief self-administered questionnaire for cluster headache screening in a tertiary center. Headache. 2009; 49:64–70. PMID: 19133334.
Article15. Torelli P, Beghi E, Manzoni GC. Validation of a questionnaire for the detection of cluster headache. Headache. 2005; 45:644–652. PMID: 15953296.
Article16. Katsarava Z, Obermann M, Yoon MS, Dommes P, Kuznetsova J, Weimar C, et al. Prevalence of cluster headache in a population-based sample in Germany. Cephalalgia. 2007; 27:1014–1019. PMID: 17666085.
Article17. Headache Classification Committee of the International Headache Society (IHS). The International Classification of Headache Disorders, 3rd edition (beta version). Cephalalgia. 2013; 33:629–808. PMID: 23771276.18. Preacher KJ, MacCallum RC. Exploratory factor analysis in behavior genetics research: factor recovery with small sample sizes. Behav Genet. 2002; 32:153–161. PMID: 12036113.19. Lipton RB, Dodick D, Sadovsky R, Kolodner K, Endicott J, Hettiarachchi J, et al. A self-administered screener for migraine in primary care: the ID Migraine validation study. Neurology. 2003; 61:375–382. PMID: 12913201.21. Lalkhen AG, McCluskey A. Clinical tests: sensitivity and specificity. Contin Educ Anaesth Crit Care Pain. 2008; 8:221–223.
Article22. McGee S. Simplifying likelihood ratios. J Gen Intern Med. 2002; 17:646–649. PMID: 12213147.
Article23. Kim BK, Chu MK, Lee TG, Kim JM, Chung CS, Lee KS. Prevalence and impact of migraine and tension-type headache in Korea. J Clin Neurol. 2012; 8:204–211. PMID: 23091530.
Article24. Lin KH, Wang PJ, Fuh JL, Lu SR, Chung CT, Tsou HK, et al. Cluster headache in the Taiwanese–a clinic-based study. Cephalalgia. 2004; 24:631–638. PMID: 15265051.25. Voiticovschi-Iosob C, Allena M, De Cillis I, Nappi G, Sjaastad O, Antonaci F. Diagnostic and therapeutic errors in cluster headache: a hospital-based study. J Headache Pain. 2014; 15:56. PMID: 25178541.
Article26. Barbanti P, Fabbrini G, Pesare M, Vanacore N, Cerbo R. Unilateral cranial autonomic symptoms in migraine. Cephalalgia. 2002; 22:256–259. PMID: 12100086.
Article27. Barbanti P, Aurilia C, Dall'Armi V, Egeo G, Fofi L, Bonassi S. The phenotype of migraine with unilateral cranial autonomic symptoms documents increased peripheral and central trigeminal sensitization. A case series of 757 patients. Cephalalgia. 2016; 36:1334–1340. PMID: 26858260.
Article28. Lai TH, Fuh JL, Wang SJ. Cranial autonomic symptoms in migraine: characteristics and comparison with cluster headache. J Neurol Neurosurg Psychiatry. 2009; 80:1116–1119. PMID: 18931007.
Article29. Obermann M, Yoon MS, Dommes P, Kuznetsova J, Maschke M, Weimar C, et al. Prevalence of trigeminal autonomic symptoms in migraine: a population-based study. Cephalalgia. 2007; 27:504–509. PMID: 17428298.
Article30. Taga A, Russo M, Manzoni GC, Torelli P. Cluster headache with accompanying migraine-like features: a possible clinical phenotype. Headache. 2017; 57:290–297. PMID: 27861832.
Article31. Torelli P, Beghi E, Manzoni GC. Cluster headache prevalence in the Italian general population. Neurology. 2005; 64:469–474. PMID: 15699377.
Article32. Katsarava Z, Dzagnidze A, Kukava M, Mirvelashvili E, Djibuti M, Janelidze M, et al. Prevalence of cluster headache in the Republic of Georgia: results of a population-based study and methodological considerations. Cephalalgia. 2009; 29:949–952. PMID: 19250289.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Acute Lymphocytic Leukemia of Periorbital Tissue Presenting with Cluster-Like Headache
- Prevention with 240 mg Galcanezumab of Episodic Cluster Headache
- Cluster-like Headache Secondary to Cerebral Venous Thrombosis
- Pulsed Radiofrequency of the Sphenopalatine Ganglion for Treatment of a Cluster Headache: A case report
- Cluster Headache Presenting With Raeder's Syndrome