Blood Res.
2018 Jun;53(2):117-122. 10.5045/br.2018.53.2.117.
Impact of rituximab and half-dose CHOP as primary therapy for untreated symptomatic Waldenström Macroglobulinemia: review of a combined regimen of rituximab with an alkylating agent
- Affiliations
-
- 1Hematology Division, National Hospital Organization Disaster Medical Center, Tokyo, Japan. nao26nao26@gmail.com
- 2Clinical Research Division, National Hospital Organization Disaster Medical Center, Tokyo, Japan.
- 3Pharmaceutical Division, National Hospital Organization Disaster Medical Center, Tokyo, Japan.
- 4Laboratory and Pathology Division, National Hospital Organization Disaster Medical Center, Tokyo, Japan.
- 5Clinical Oncology Division, National Hospital Organization Disaster Medical Center, Tokyo, Japan.
- KMID: 2451029
- DOI: http://doi.org/10.5045/br.2018.53.2.117
Abstract
- BACKGROUND
Waldenström Macroglobulinemia (WM) is a rare subtype of indolent B-cell lymphoma, and prospective randomized studies on WM are scarce. The R-CHOP therapy [rituximab (R), cyclophosphamide, hydroxy-doxorubicin, vincristine, and prednisone] is a popular and recommended regimen for primary therapy, prescribed by several treatment guidelines for WM. However, treatment with R-CHOP is accompanied by severe myelosuppression and high rates of peripheral neuropathy. Therefore, we retrospectively evaluated the efficacy and toxicity of half-dose CHOP combined with R as a primary therapy for WM.
METHODS
Patients with untreated symptomatic WM, treated at the Disaster Medical Center between April 2011 and September 2016, were retrospectively analyzed after administration of 6 cycles of half-dose R-CHOP for every 3 weeks. The response, median time to response, best response, progression-free survival, overall survival, and toxicities were evaluated.
RESULTS
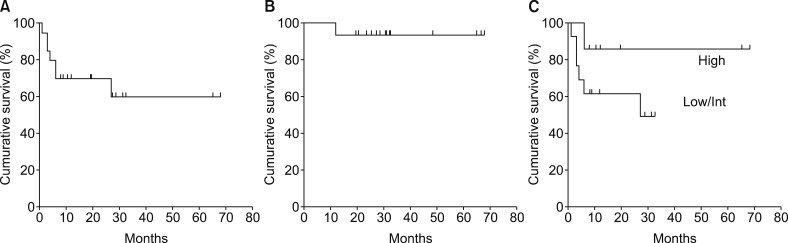
Of the 20 WM patients analyzed, 16 (80%) received half-dose R-CHOP without vincristine, and 13 (65%) responded to the treatment. With a median follow-up duration of 26.3 months, the 2-year progression-free survival and 2-year overall survival rates were 70 and 93.3%, respectively. The median time to response and best response were 6 and 9.9 weeks, respectively. Grade 3/4 leukocytopenia, neutropenia, febrile neutropenia, and Grade 1 peripheral neuropathy developed in 32, 37, 0, and 21% of patients, respectively.
CONCLUSION
The half-dose R-CHOP is an effective and well-tolerated primary therapy for WM. To the best of our knowledge, this is the first study reporting the use of a reduced-dose R-CHOP regimen for the primary treatment of WM.
MeSH Terms
Figure
Cited by 1 articles
-
What is the most appropriate regimen for untreated Waldenström macroglobulinemia? - An updated analysis of rituximab and half-dose CHOP therapy and cost effectiveness
Naohiro Sekiguchi, Airi Hamano, Tomoko Kitagawa, Kenichi Ito, Kazuhiko Hirano, Kazuaki Yamada
Blood Res. 2019;54(2):153-156. doi: 10.5045/br.2019.54.2.153.
Reference
-
1. Swerdlow SH, Berger F, Pikeru SA. Lymphoplasmacytic lymphoma. In : Swerdlow SH, Campo E, Harris NL, editors. WHO classification of tumour of hematopoietic and lymphoid tissues. 4th ed. Lyon, France: IARC Press;2007. p. 194–195.2. Owen RG, Treon SP, Al-Katib A, et al. Clinicopathological definition of Waldenstrom's macroglobulinemia: consensus panel recommendations from the Second International Workshop on Waldenstrom's Macroglobulinemia. Semin Oncol. 2003; 30:110–115. PMID: 12720118.
Article3. Issa GC, Leblebjian H, Roccaro AM, Ghobrial IM. New insights into the pathogenesis and treatment of Waldenstrom macroglobulinemia. Curr Opin Hematol. 2011; 18:260–265. PMID: 21519243.
Article4. Vijay A, Gertz MA. Waldenström macroglobulinemia. Blood. 2007; 109:5096–5103. PMID: 17303694.
Article5. Buske C, Hoster E, Dreyling M, et al. The addition of rituximab to front-line therapy with CHOP (R-CHOP) results in a higher response rate and longer time to treatment failure in patients with lymphoplasmacytic lymphoma: results of a randomized trial of the German Low-Grade Lymphoma Study Group (GLSG). Leukemia. 2009; 23:153–161. PMID: 18818699.
Article6. Rummel MJ, Niederle N, Maschmeyer G, et al. Bendamustine plus rituximab versus CHOP plus rituximab as first-line treatment for patients with indolent and mantle-cell lymphomas: an open-label, multicentre, randomised, phase 3 non-inferiority trial. Lancet. 2013; 381:1203–1210. PMID: 23433739.
Article7. Leblond V, Johnson S, Chevret S, et al. Results of a randomized trial of chlorambucil versus fludarabine for patients with untreated Waldenström macroglobulinemia, marginal zone lymphoma, or lymphoplasmacytic lymphoma. J Clin Oncol. 2013; 31:301–307. PMID: 23233721.
Article8. National Cancer Institute. NCCN Clinical Practice Guidelines in Oncology. Waldenstrom macroglobulinemia/lymphoplasmacytic lymphoma. Bethesda, MD: National Cancer Institute;2017. Accessed August 3, 2017. at http://www.nccn.org/professionals/physician_gls/f_guidelines.asp.9. Buske C, Leblond V, Dimopoulos M, et al. Waldenstrom's macroglobulinaemia: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann Oncol. 2013; 24:vi155–vi159. PMID: 24078658.10. Dimopoulos MA, Kastritis E, Owen RG, et al. Treatment recommendations for patients with Waldenström macroglobulinemia (WM) and related disorders: IWWM-7 consensus. Blood. 2014; 124:1404–1411. PMID: 25027391.
Article11. Treon SP. How I treat Waldenström macroglobulinemia. Blood. 2015; 126:721–732. PMID: 26002963.
Article12. Ioakimidis L, Patterson CJ, Hunter ZR, et al. Comparative outcomes following CP-R, CVP-R, and CHOP-R in Waldenström's macroglobulinemia. Clin Lymphoma Myeloma. 2009; 9:62–66. PMID: 19362976.
Article13. Coiffier B, Lepage E, Briere J, et al. CHOP chemotherapy plus rituximab compared with CHOP alone in elderly patients with diffuse large-B-cell lymphoma. N Engl J Med. 2002; 346:235–242. PMID: 11807147.
Article14. Nobile-Orazio E, Marmiroli P, Baldini L, et al. Peripheral neuropathy in macroglobulinemia: incidence and antigen-specificity of M proteins. Neurology. 1987; 37:1506–1514. PMID: 2442666.
Article15. Nemni R, Gerosa E, Piccolo G, Merlini G. Neuropathies associated with monoclonal gammapathies. Haematologica. 1994; 79:557–566. PMID: 7503840.16. Morel P, Duhamel A, Gobbi P, et al. International prognostic scoring system for Waldenstrom macroglobulinemia. Blood. 2009; 113:4163–4170. PMID: 19196866.17. Treon SP, Merlini G, Morra E, Patterson CJ, Stone MJ. Report from the Sixth International Workshop on Waldenström's Macroglobulinemia. Clin Lymphoma Myeloma Leuk. 2011; 11:69–73.
Article18. Kyle RA, Rajkumar SV. Criteria for diagnosis, staging, risk stratification and response assessment of multiple myeloma. Leukemia. 2009; 23:3–9. PMID: 18971951.
Article19. Cancer Therapy Evaluation Program (CTEP). Common Terminology Criteria for Adverse Events ver4.0. Bethesda, MD: National Cancer Institute;2010. Accessed August 3, 2017. at http://ctep.cancer.gov/protocolDevelopment/electronic_applications/ctc.htm#ctc_40.20. Dimopoulos MA, Zervas C, Zomas A, et al. Treatment of Waldenström's macroglobulinemia with rituximab. J Clin Oncol. 2002; 20:2327–2333. PMID: 11981004.
Article21. Gertz MA, Abonour R, Heffner LT, Greipp PR, Uno H, Rajkumar SV. Clinical value of minor responses after 4 doses of rituximab in Waldenström macroglobulinaemia: a follow-up of the Eastern Cooperative Oncology Group E3A98 trial. Br J Haematol. 2009; 147:677–680. PMID: 19751237.
Article22. Marcus R, Imrie K, Solal-Celigny P, et al. Phase III study of R-CVP compared with cyclophosphamide, vincristine, and prednisone alone in patients with previously untreated advanced follicular lymphoma. J Clin Oncol. 2008; 26:4579–4586. PMID: 18662969.
Article23. Hiddemann W, Kneba M, Dreyling M, et al. Frontline therapy with rituximab added to the combination of cyclophosphamide, doxorubicin, vincristine, and prednisone (CHOP) significantly improves the outcome for patients with advanced-stage follicular lymphoma compared with therapy with CHOP alone: results of a prospective randomized study of the German Low-Grade Lymphoma Study Group. Blood. 2005; 106:3725–3732. PMID: 16123223.
Article24. Buske C, Sadullah S, Kastritis E, et al. Generation of a large observational pan-European data platform for treatment and outcome patterns in patients with Waldenstrom's macroglobulinemia. Blood (ASH Annual Meeting Abstracts). 2015; 126(Suppl):abst 2096.
Article25. Olszewski AJ, Fallah J, Eaton CB, Treon SP, Castillo JJ. The evolution of management and survival outcomes of Waldenström macroglobulinemia (WM) in the United States (US). Blood (ASH Annual Meeting Abstracts). 2015; 126(Suppl):abst 882.
Article26. Olszewski AJ, Treon SP, Castillo JJ. Evolution of management and outcomes in Waldenström macroglobulinemia: a population-based analysis. Oncologist. 2016; 21:1377–1386.
Article27. Dimopoulos MA, Anagnostopoulos A, Kyrtsonis MC, et al. Primary treatment of Waldenström macroglobulinemia with dexamethasone, rituximab, and cyclophosphamide. J Clin Oncol. 2007; 25:3344–3349. PMID: 17577016.
Article28. Kikuchi M, Nakasone H, Akahoshi Y, et al. Reduced-dose (two-thirds) R-CHOP chemotherapy for elderly patients with non-Hodgkin lymphoma. J Chemother. 2015; 27:99–105. PMID: 25314911.
Article29. Peyrade F, Jardin F, Thieblemont C, et al. Attenuated immunochemotherapy regimen (R-miniCHOP) in elderly patients older than 80 years with diffuse large B-cell lymphoma: a multicentre, single-arm, phase 2 trial. Lancet Oncol. 2011; 12:460–468. PMID: 21482186.30. Dimopoulos MA, García-Sanz R, Gavriatopoulou M, et al. Primary therapy of Waldenstrom macroglobulinemia (WM) with weekly bortezomib, low-dose dexamethasone, and rituximab (BDR): long-term results of a phase 2 study of the European Myeloma Network (EMN). Blood. 2013; 122:3276–3282. PMID: 24004667.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- What is the most appropriate regimen for untreated Waldenström macroglobulinemia? - An updated analysis of rituximab and half-dose CHOP therapy and cost effectiveness
- A Case of Mantle Cell Lymphoma Treated with Autologous Stem Cell Transplantation and Rituximab
- Rituximab Plus CHOP for the Treatment of Primary Mediastinal Large B Cell Lymphoma in a Pregnant Woman
- Addition of rituximab to the CHOP regimen has no benefit in patients with primary extranodal diffuse large B-cell lymphoma
- A Case of Waldenstrom's Macroglobulinemia Treated with 2-Chlorodeoxyadenosine (Cladribine)